Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Although glucocorticoids (GCs) may be appropriate in rheumatoid arthritis (RA), there is general agreement that GCs sparing is desirable. The safety of GCs is duration and dose dependent and the risk has been demonstrated for a dose ≥ 5 mg/day. Tocilizumab (TCZ) is an effective biologic therapy in RA, even without concomitant methotrexate. The aim of this study is to describe the GCs sparing effect in patients treated with TCZ in real life clinical setting.
Methods:
Multicenter, prospective, non-interventional 12-month study. Patients had active RA, were treated with more than 5 mg daily of prednisone for at least 3 months and initiated TCZ. No instructions were given regarding TCZ or GCs doses. Patient’s characteristics were collected at baseline. After TCZ initiation, monthly disease activity components and RA treatments doses were collected. HAQ-DI and RAID were completed every 6 months. Primary endpoint: proportion of patients treated by 5mg/day or less of prednisone at M12 without intensification of synthetic DMARDs. Analysis: patients fulfilling inclusion/non-inclusion criteria and with at least one TCZ infusion. For primary endpoint, patients with missing GCs dose at M12 or with missing data on DMARDs intensification were considered as “non-responders”. Last Observation Carried Forward (LOCF) method was used for missing cortisone dose value.
Results:
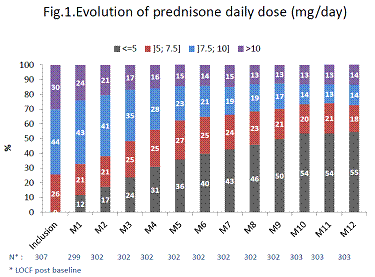
321 patients were selected and 307 analyzed. Baseline characteristics were: mean age 57±14 years, 249 females (78%), disease duration 10±9 years, RF or anti-CCP positive: 249 (82%); DAS 28-ESR: 5.1±1.3; CDAI: 27±12; SDAI: 29±13; HAQ: 1.6±0.7; RAID: 6.2±2.0; 216 (71%) previously treated by biologic DMARD. TCZ was initiated as monotherapy in 116 (38%) patients. At TCZ initiation, 92 (30%) patients received more than 10 mg of prednisone daily, 136 (44%) 7.5 to 10 mg, 79 (26%) 5 to 7.5 mg with a mean dose of 12±7 mg/day. At M12, 185 (66%) patients completed the study. 105 (34%) patients prematurely withdrew for: adverse event (AE) (n=38), TCZ discontinuation (n=47), lost-to-follow-up/move/refusal to continue the study (n=19). One 82-year old patient died after osteoporotic fracture. At M12, 124 patients (40%, 95%CI [35%; 46%]) received 5 mg or less of prednisone/day without DMARD intensification and 20% have stopped GCs. 33 % were in DAS28 remission and 42% had low disease activity. Mean DAS 28-ESR, CDAI and SDAI were 2.3±1.2, 9.3±8.4 and 9.7±8.5 respectively. At M12, 14% received prednisone daily dose>10 mg, 14% 7.5 to 10 mg, 18% 5 to 7.5 mg and 55% received 5 mg or less (Fig.1). No new safety signal was reported. 211 (67%) patients had at least one AE, 44 (14%) had at least one serious AE.
Conclusion:
This study is the first large prospective study evaluating TCZ GCs sparing effect in RA in real life. 40% of patients were able to decrease their prednisone dose to 5 mg or below at 12 months without increasing disease activity.
Disclosure:
A. Saraux,
Roche Pharmaceuticals,
2,
Chugai Pharma,
5,
Merck Sharp Dohme-Chibret,
5,
Bristol-Myers Squibb,
5,
Abbvie,
5,
UCB Pharma,
5;
S. Rouanet,
Roche Pharmaceuticals,
3;
R. M. Flipo,
Roche Pharmaceuticals,
5,
Chugai Pharma,
5,
Merck Sharp & Dohme-Chibret,
5,
Abbvie,
5,
UCB Pharma,
5;
J. C. Poncet,
Roche Pharmaceuticals,
2,
Chugai Pharma,
2;
P. Fardellone,
Roche Pharmaceuticals,
2,
Chugai Pharma,
2;
P. Hilliquin,
Roche Pharmaceuticals,
5,
Chugai Pharma,
5,
Bristol-Myers Squibb,
5,
Merck Sharp & Dohme-Chibret,
5,
UCB Pharma,
5,
Pfizer Inc,
5,
Expanscience,
5;
I. Idier,
Chugai,
3;
A. G. Cantagrel,
Roche Pharmaceuticals,
5,
Chugai Pharma,
5,
Merck Sharp & Dohme-Chibret,
5,
Bristol-Myers Squibb,
5,
Abbvie,
5,
UCB Pharma,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-glucocorticoids-sparing-effect-the-spare-1-study/