Session Information
Date: Sunday, November 17, 2024
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The GiACTA trial, a phase III global trial of tocilizumab (TCZ) for giant cell arteritis (GCA), did not involve Japanese patients, and real-world data on TCZ for GCA in Japan are limited. This study aims to evaluate the effectiveness and safety of TCZ for GCA in routine clinical practice in Japan.
Methods: This multicenter study retrospectively identified patients with GCA who initiated TCZ at seven hospitals in Japan from January 2008 to July 2021. Rheumatologists at each institution clinically diagnosed GCA according to the 1990 ACR criteria. The patients were followed for 2 years after TCZ initiation, and we excluded patients whose follow-up was < 6 months.
We collected information regarding patient demographics, GCA symptoms at diagnosis, diagnostic methods, and treatment details at TCZ initiation. GCA relapses, TCZ-related adverse events, and treatment adjustments were monitored during the 2-year follow-up. This study descriptively summarized these data.
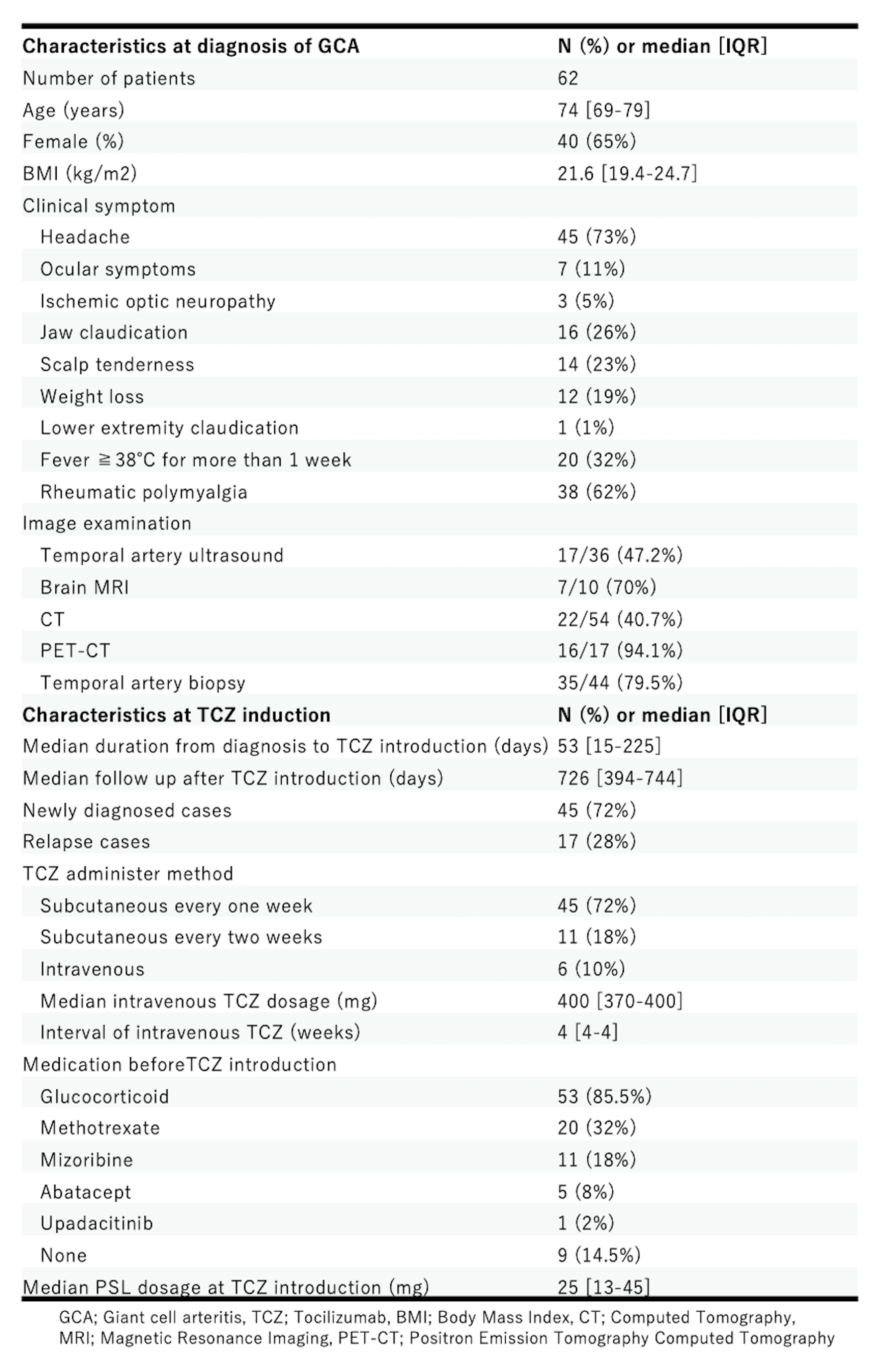
Results: A total of 62 GCA patients were identified, with a median age of 74 years (IQR; 69-79), and 65% were female (Table 1). Headache (73%), polymyalgia rheuamtica (62%), and fever (32%) were frequent symptoms at GCA diagnosis. TCZ was administered subcutaneously every week in 72%, every two weeks in 18%, and intravenously in 10% of the patients.
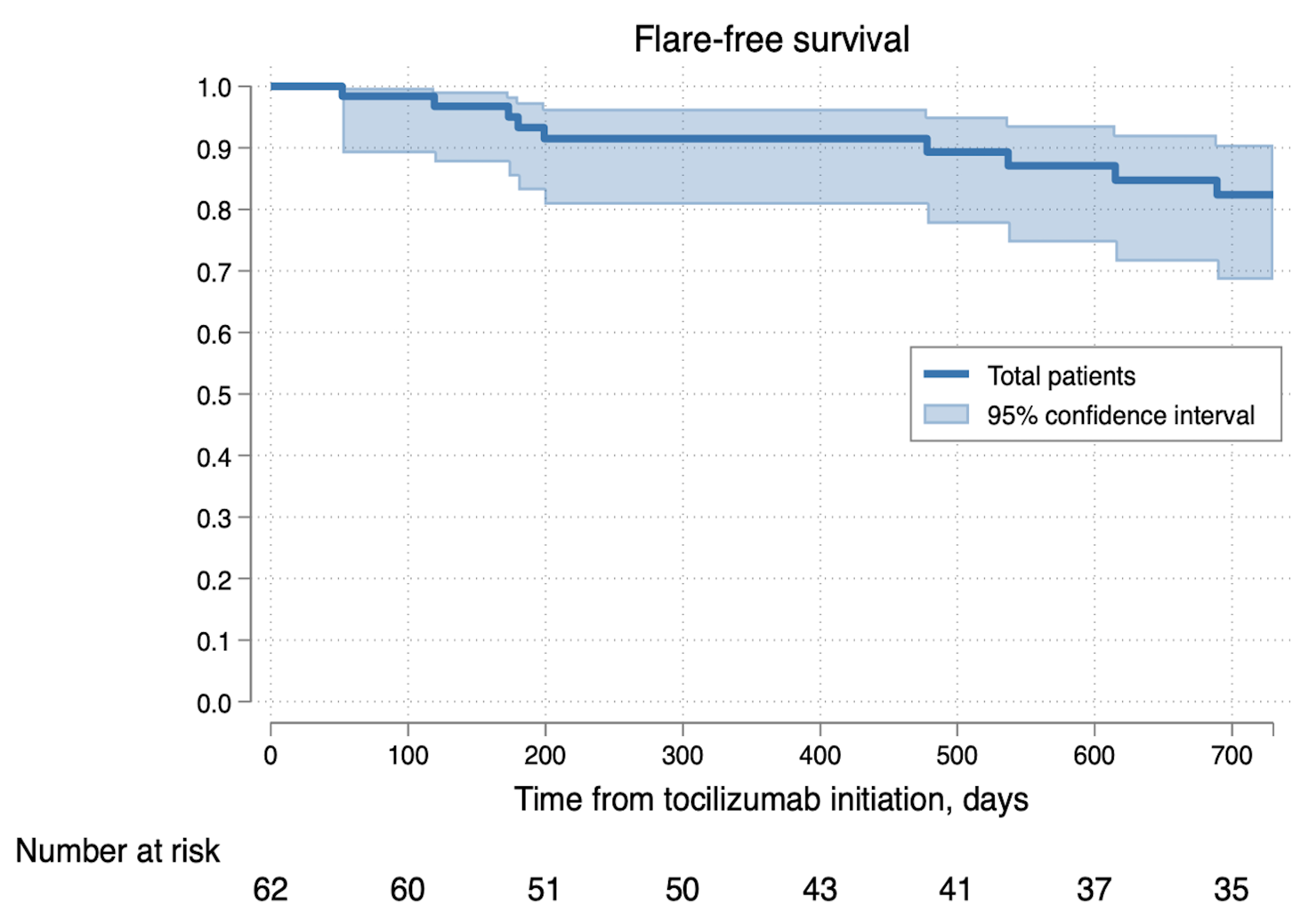
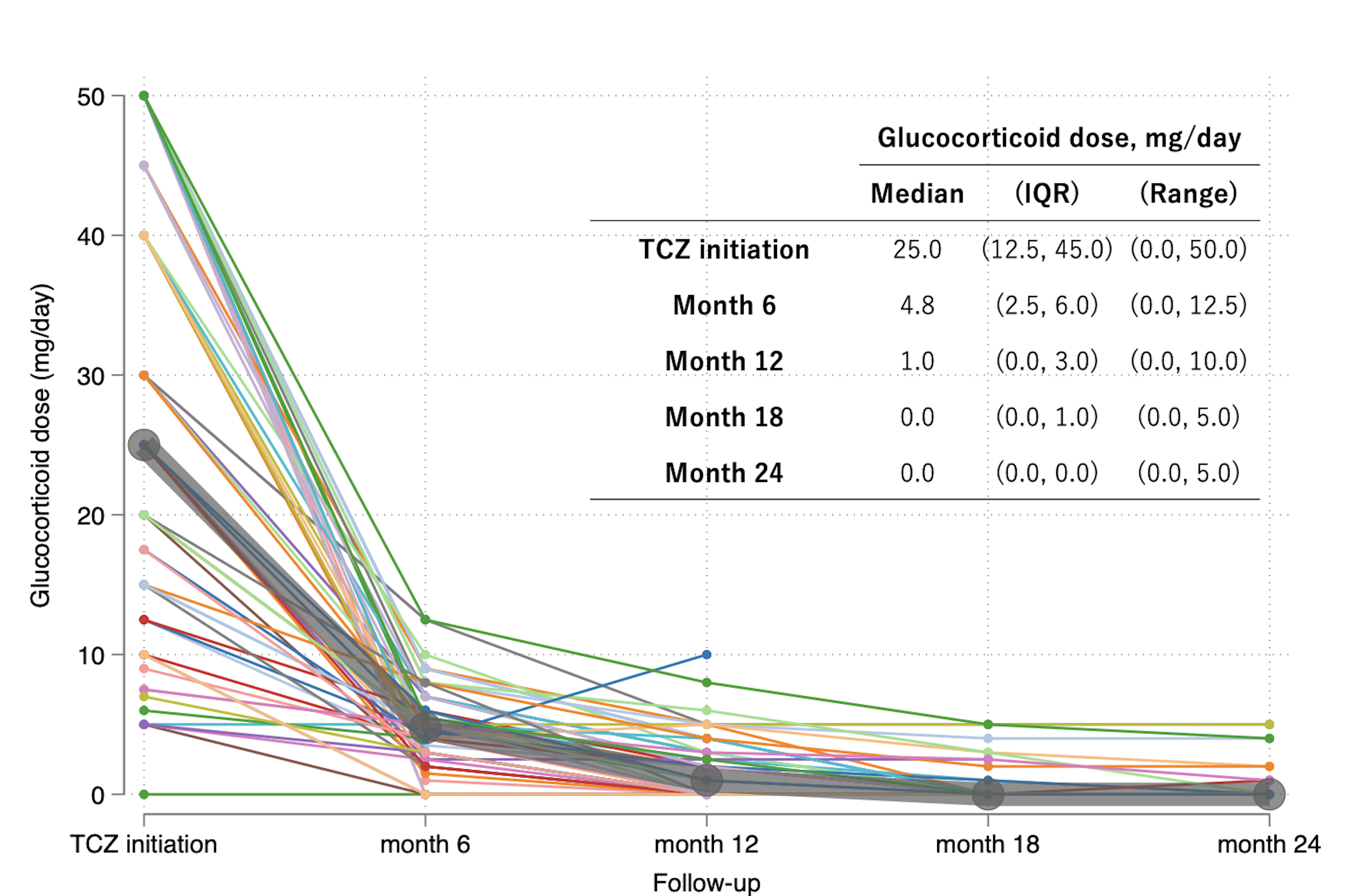
Following TCZ administration, patients were followed for a median of 726 days (IQR; 394-744), and 10 cases (16%) relapsed; 2 cases were on full-dose TCZ, and the remaining 8 cases relapsed after discontinuing or extending the dose intervals of TCZ (Figure1). GC doses decreased over 2 years. GC discontinuation was achieved in 11.3% (7/62) at month 6, 44.4% (24/54) at month 12, 72.3% (34/47) at month 18, and 79.1% (34/43) at month 24 (Figure2). In patients who received intravenous TCZ, 5/6 patients achieved GC-free remission at the last observation, and all patients achieved GC-free remission at month 24. A total of 29 adverse events were reported in 23 patients (37%). The main adverse events were severe and non-severe infections, and 8 adverse events (13%) required the discontinuation of TCZ. Of these, 2 were severe infections, 1 was a non-severe infection, 1 was an injection site reaction, and 4 were other adverse events, including gastrocnemius pain, elevated liver enzymes, general fatigue, and decreased platelet count.
Conclusion: This multicenter observational study demonstrated the effectiveness and safety of TCZ over 2 years in Japanese patients with GCA. In the real-world setting, many patients could discontinue GC, and most relapses occurred in patients who discontinued or spaced doses of TCZ. There were few serious infections requiring discontinuation of TCZ.
Lines showed trajectories of glucocorticoid doses (prednisolone equivalent) in each patient. A thick black line showed median dose of glucocorticoid. TCZ, tocilizumab
To cite this abstract in AMA style:
Oda N, Fukui S, Yamaguchi T, Nagase F, Ito T, Watanabe M, Haji Y, Suyama Y, Nomura A, Uechi E, Suda M, Takizawa N, Rokutanda R, Tamaki H. Tocilizumab for Giant Cell Arteritis in Japan over 2 Years: A Multicenter Retrospective Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/tocilizumab-for-giant-cell-arteritis-in-japan-over-2-years-a-multicenter-retrospective-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-for-giant-cell-arteritis-in-japan-over-2-years-a-multicenter-retrospective-study/