Session Information
Date: Sunday, November 10, 2019
Title: 3S032: Plenary I (805–809)
Session Type: Plenary Session I
Session Time: 11:00AM-12:30PM
Background/Purpose: Pulmonary arterial hypertension (PAH) is a severe cardiopulmonary disease characterized by an obliterative vasculopathy and vascular remodeling, right heart hypertrophy, and premature death. Connective tissue disease associated PAH (CTD-PAH) is common in scleroderma, and other autoimmune diseases, which have poor clinical outcomes. Recently, it has been shown that female human TNF-transgenic (TNF-Tg) mice die by 6-months from cardiopulmonary disease. Thus, we aimed to formally characterize this pathophysiology and assess its potential as a model of CTD-PAH.
Methods: Histologic analysis and immunofluorescent (IF) staining was performed on female TNF-Tg (3647 line) and wild type (WT) mice to characterize the pulmonary vascular and right ventricular pathology. Mice (n > 4) underwent: right heart catheterization to assess hemodynamics, or barium-perfused micro-CT to assess vascular morphology, or gas chromatography. Lungs from TNF-Tg/WT bone marrow chimeric mice, and anti-TNF vs. placebo treated TNF-Tg mice were assessed (n > 3). RNA sequencing was performed on lung tissue, and bioinformatic techniques were applied to compare TNF-Tg mouse lungs to publicly available human normal and CTD-PAH transcriptomic data.
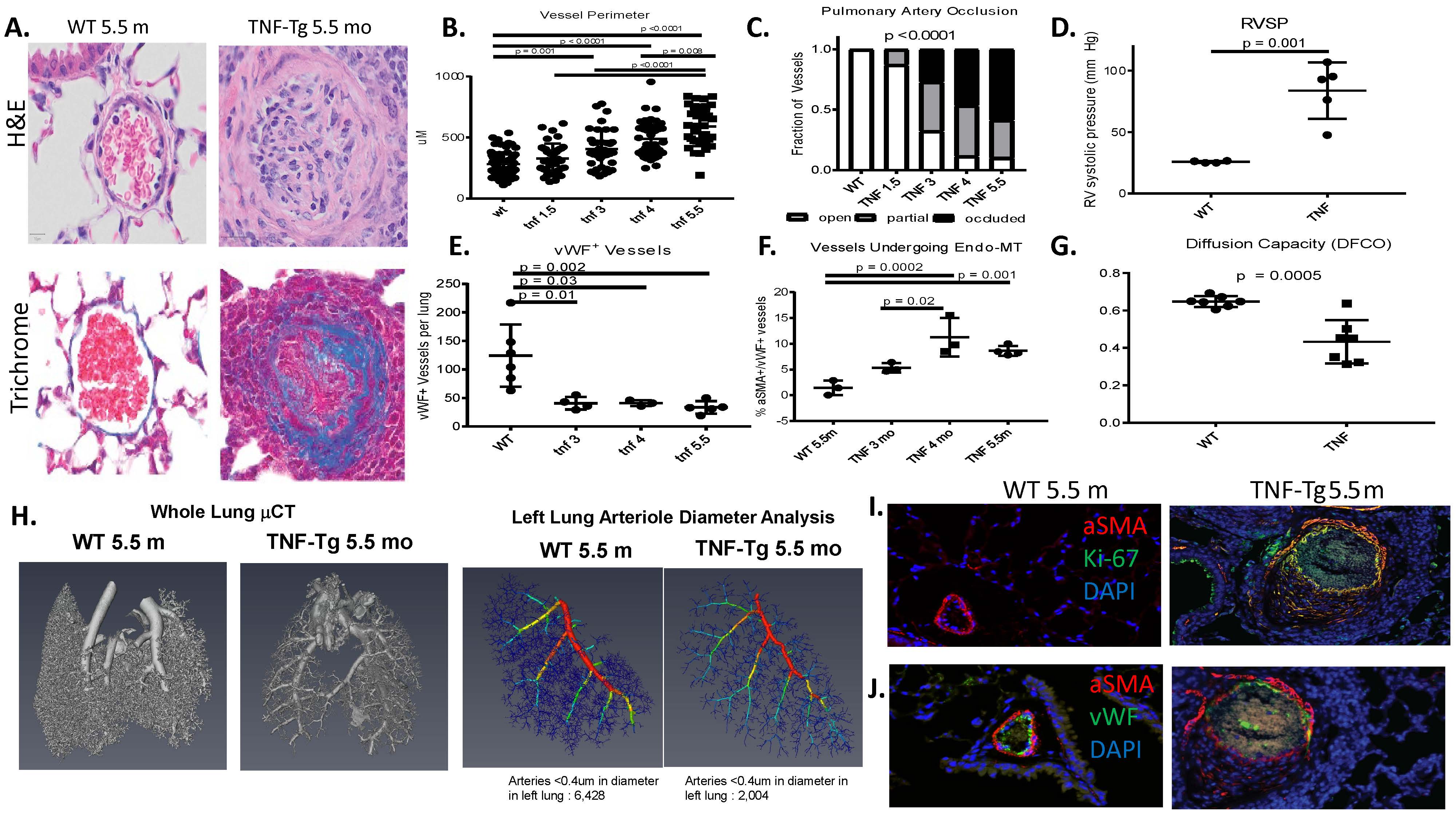
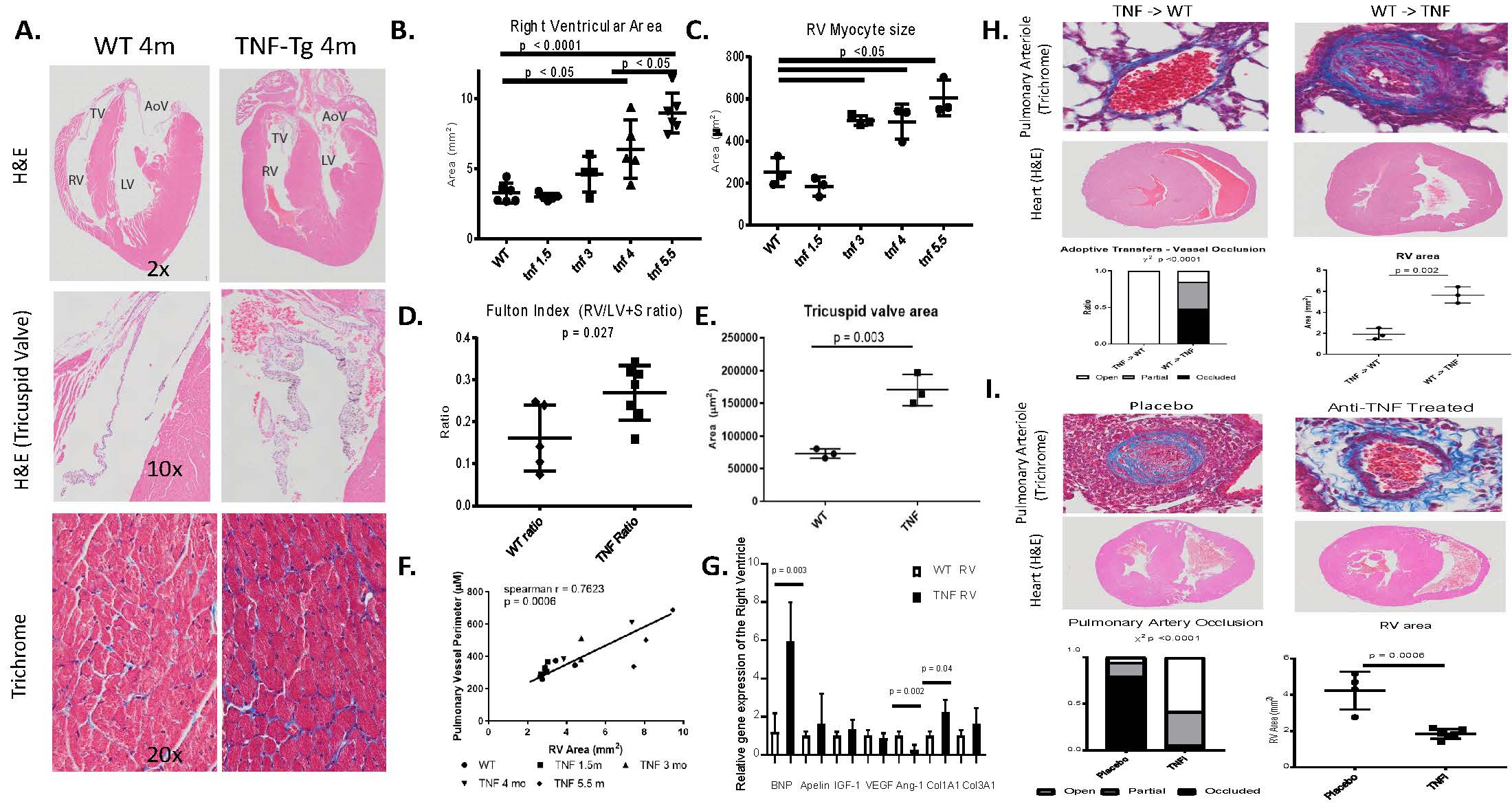
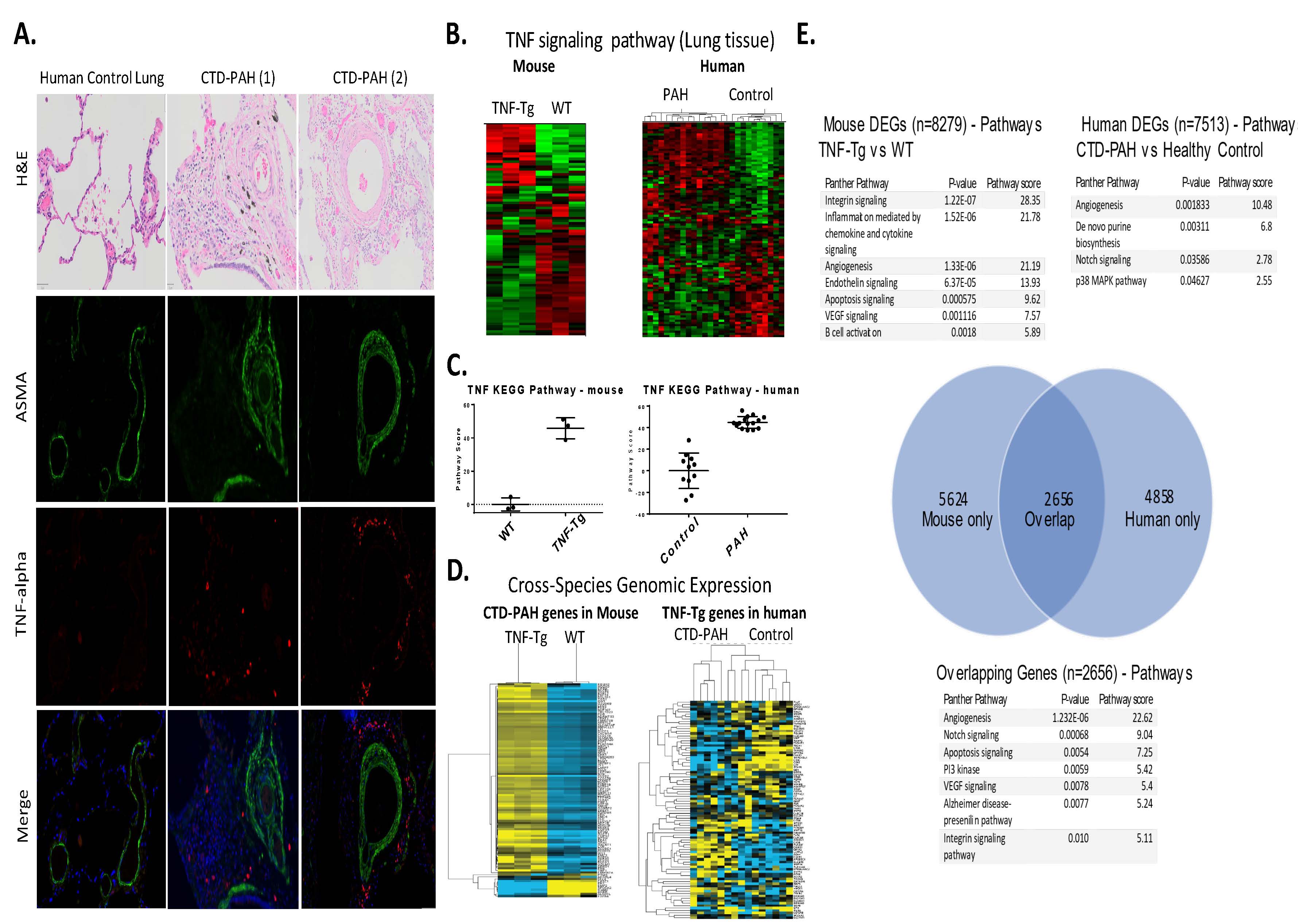
Results: Female TNF-Tg mice display a progressive pulmonary vasculopathy beginning at 3 months of age manifested by vascular collagen deposition, enlarged pulmonary arteries, attenuation of distal arterioles, and vascular occlusion, which closely resemble CTD-PAH pathologically (Fig 1A-C). Hemodynamic assessment demonstrated a significantly increased right ventricular systolic pressure of 83.7± 10.3 mmHg vs. 25.7 ± 0.4 mmHg in TNF-Tg vs. WT mice (Fig 1D), making this one of the most robust models of murine PAH ever reported. μCT analysis confirmed pruning of the vascular tree (Fig 1H), and TNF-Tg mice had reduced gas exchange (Fig 1G). Increased aSMA IF staining in TNF-Tg lungs corresponded to proliferation (Ki-67+), and loss of von Willebrand factor positive (vWF+) vessels over time. We also observed an increase in aSMA+vWF+ cells (Fig 1, E, F, I), implicating endothelial-mesenchymal transition in this process. By 4 months of age, TNF-Tg mice display remarkable right ventricular hypertrophy, and transcriptional evidence of RV dysregulation (Fig 2A-G). Bone marrow chimera experiments revealed that mesenchymal cells, and not bone-marrow derived cells, are necessary to drive this process (Fig 2H), while anti-TNF therapy halted the progression of PAH pathology (Fig 2I). Human SSc-PAH lungs display increased TNF-a staining (Fig 3A), and human microarray data demonstrated a prominent TNF signature that can distinguish PAH from control lungs (Fig 3B). Comparison of gene expression between TNF-Tg lungs and CTD-PAH lungs showed significant similarities in expression patterns and clustering (Fig 3C) with enrichment in pathway overlaps including angiogenesis, Notch signaling, apoptosis, and VEGF signaling (Fig 3D).
Conclusion: The TNF-Tg mouse represents a novel model of CTD-PAH which recapitulates nearly all key features of the disease and can serve as a valuable tool to better study and test potential CTD-PAH therapeutics.
pah acr abstract figures korman et al 1
pah acr abstract figures korman et al 2
pah acr abstract figures korman et al 3
To cite this abstract in AMA style:
Korman B, Bell R, White R, Garcia-Hernandez M, Wu E, Slattery P, Huertas N, Duemmel S, Nuzzo M, Rahimi H, Morrell C, Ritchlin C, Schwarz E. TNF-α Drives Progressive Obliterative Pulmonary Vascular Disease and Represents a Novel Model of Connective-Tissue Disease Associated Pulmonary Arterial Hypertension (CTD-PAH) [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/tnf-%ce%b1-drives-progressive-obliterative-pulmonary-vascular-disease-and-represents-a-novel-model-of-connective-tissue-disease-associated-pulmonary-arterial-hypertension-ctd-pah/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tnf-%ce%b1-drives-progressive-obliterative-pulmonary-vascular-disease-and-represents-a-novel-model-of-connective-tissue-disease-associated-pulmonary-arterial-hypertension-ctd-pah/
