Session Information
Date: Tuesday, November 7, 2017
Title: Rheumatoid Arthritis – Clinical Aspects IV: Medications and Risk
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Current guidelines recommend holding biologic DMARDs before major surgery, despite limited data. Few studies have examined perioperative timing of individual biologic therapies. This study evaluated whether holding abatacept infusions before elective hip or knee arthroplasty is associated with a decreased risk of serious post-operative infection.
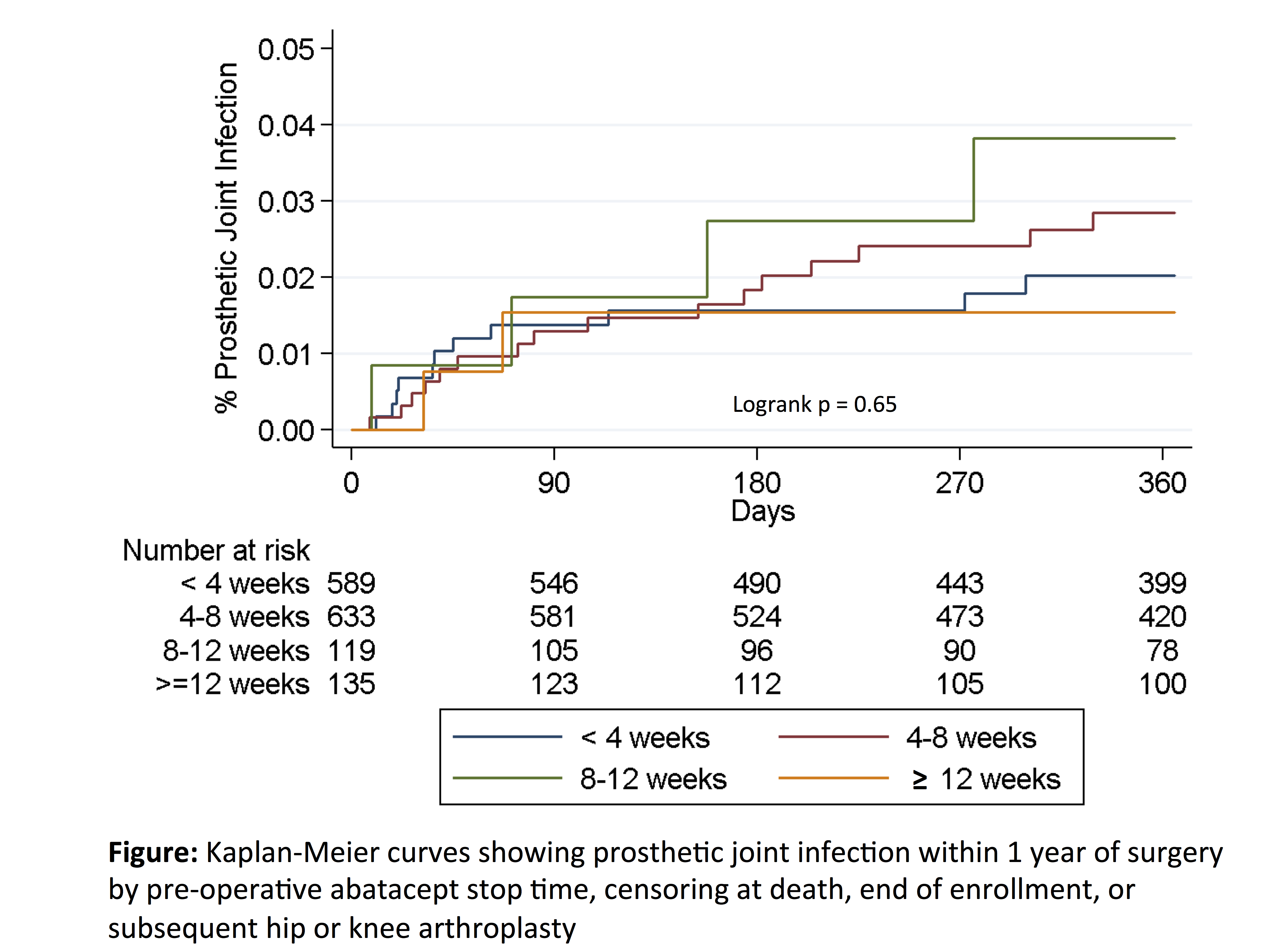
Methods: This retrospective cohort study using U.S. Medicare claims data from 2006-2014 evaluated adults with ≥ 2 ICD9 codes for RA who received abatacept by infusion (precisely dated in claims data) within 6 months of elective inpatient primary or revision total hip or knee arthroplasty. Patients with hip fracture, malignancy, pre-existing infection, non-elective procedures (admission through the emergency department or as a hospital transfer), or with surgery after hospital day 3 were excluded. Multivariable logistic regression evaluated associations of abatacept stop timing (time between most recent infusion and surgery categorized in 4 week intervals based on dosing interval) with serious (hospitalized) infection within 30 days using a validated set of discharge diagnoses from inpatient hospitalizations, adjusting for potential confounders. Associations of abatacept stop time with rate of prosthetic joint infection (PJI, ICD9 996.66) within 1 year were examined using Kaplan-Meier curves.
Results: Among 1476 surgeries in 1358 patients, serious infection within 30 days occurred in 10.1% (n = 149). Urinary infection, skin/soft tissue infection, and pneumonia were the most common infections. In adjusted analyses, stop timing of 4-8 weeks (held for one dosing interval) vs. < 4 weeks was not associated with a significant decrease in the risk of infection [OR 0.91 (0.62-1.33), p = 0.62] (Table). Glucocorticoid dose > 7.5mg/day vs. none was associated with increased risk of infection [OR 2.38 (1.42-3.98), p = 0.001]. Over 12 months, the rate of PJI was 2.7 per 100 person-years (n = 33 infections). Rates of PJI within 1 year were similar in patients with abatacept stop timing < 4 weeks vs. longer stop timing (Figure).
Conclusion: Holding abatacept for one dosing interval (i.e. one month) was not associated with a decrease in the risk of post-operative infection. In contrast, glucocorticoid dose > 7.5mg per day was associated with a substantial increase in the risk of post-operative infection. Given the potential risk of flare, holding abatacept prior to elective joint procedures may not be warranted.
To cite this abstract in AMA style:
George MD, Baker J, Winthrop K, Alemao E, Chen L, Connolly S, Simon T, Wu Q, Xie F, Yang S, Curtis JR. Timing of Abatacept Infusions before Elective Arthroplasty and the Risk of Post-Operative Infection [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/timing-of-abatacept-infusions-before-elective-arthroplasty-and-the-risk-of-post-operative-infection/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/timing-of-abatacept-infusions-before-elective-arthroplasty-and-the-risk-of-post-operative-infection/