Session Information
Date: Monday, November 6, 2017
Title: Rheumatoid Arthritis – Clinical Aspects II: Treatment Patterns
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Treatment recommendations aim to improve outcomes in rheumatoid arthritis (RA) through early identification and a treat-to-target approach. We examined recent trends over the past decade including patient characteristics, treatment strategies and disease activity in the first year of follow-up in a large Canadian early inflammatory arthritis (EIA) cohort.
Methods: Data were from individuals with EIA (RA or probably RA <1-year of symptom duration) enrolled in an ongoing prospective multi-center cohort study between 2007 and 2016 and had complete DAS28 measures at 6 and 12-months. Protocolized visits include clinical assessments, questionnaires, and laboratory investigations every 3 months for the first year. Treatment is at the discretion of the treating rheumatologist, and cohort investigators met annually to discuss means to improve outcomes. We examined trends in patient characteristics, early treatment strategies with conventional(cs) and biologic(b) DMARDs and disease activity outcomes over 12-months (12M). Multivariable logistic regression was used to identify predictors of failure to achieve low disease activity (LDA) or remission (REM) by 12M.
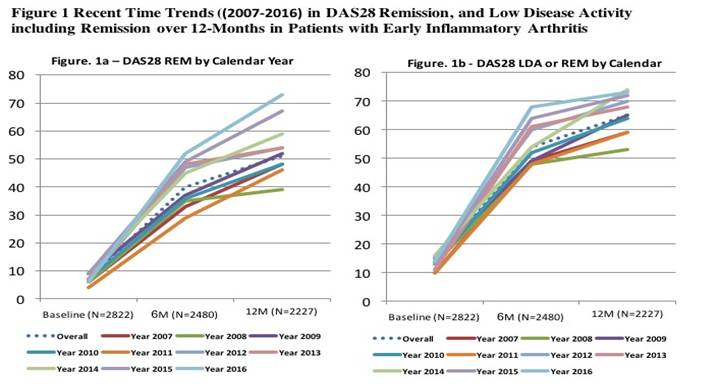
Results: Over 10 years, 2822 EIA patients were enrolled who were mostly female (70%) and Caucasian (80%). At study entry, 86% met 1987 or 2010 ACR/ EULAR RA criteria; mean(sd) age was 54(15), symptom duration was 6(3) months. Most were treated with csDMARDs (92%), often with MTX, as monotherapy or in combination with csDMARDs (77%); 29% were prescribed oral steroids and 27% IA or IM steroids. Baseline patient characteristics changed slightly over time with increases in age, male sex, education, income, and declines in current smoking (all p’s <0.01). Baseline obesity rates, comorbidities and RA characteristics (serology, inflammatory markers, joint counts and disease activity indices) remained stable. Most (87%) entered in moderate or high disease activity (MDA or HDA), and disease activity at 6 and 12M markedly improved over calendar time (Figure 1). DAS28 REM at 12M increased by over 30% (range 39% to 73%, p<0.0001), and 20% more achieved LDA or REM by 6M (range 48% to 68%, p<0.0001) (Figure 1). Time trends in treatment showed earlier titration of MTX dosing to 20mg+, increases in subcut vs. oral MTX, earlier MTX combo therapies and more rapid escalation to bDMARDs all p’s <0.05). Older age, female sex, lower education, non-white, overweight/obese BMI, and more comorbidities were associated with an inadequate response (persistent MDA or HDA) at 12 months (all p’s<0.05).
Conclusion: This study of Canadian EIA patients over time suggests that earlier, more intensified treatment promoted in practice recommendations has resulted in lower disease activity and a greater proportion of patients reaching the target of therapy. Nevertheless 25-30% of patients still did not achieve LDA or REM by 12M.
To cite this abstract in AMA style:
Schieir O, Valois MF, Bartlett SJ, Hitchon CA, Pope JE, Boire G, Haraoui B, Tin D, Thorne C, Keystone EC, Bykerk VP. Time Trends over a Decade Show Earlier Intensified Medication Strategies and Improved Outcomes in Canadians with Early Inflammatory Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/time-trends-over-a-decade-show-earlier-intensified-medication-strategies-and-improved-outcomes-in-canadians-with-early-inflammatory-arthritis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/time-trends-over-a-decade-show-earlier-intensified-medication-strategies-and-improved-outcomes-in-canadians-with-early-inflammatory-arthritis/