Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Tocilizumab (TCZ) administered subcutaneously every week (QW) or every other week (Q2W) with 26-week prednisone tapering was superior to placebo (PBO) plus 26-week (PBO+26) or 52-week (PBO+52) prednisone tapering for achievement of sustained glucocorticoid-free remission in patients with giant cell arteritis (GCA) in part 1 of the 52-week, double-blind GiACTA trial.1 In part 1, among patients with new-onset GCA at baseline, TCZ QW and TCZ Q2W treatment reduced the risk for GCA flare compared with PBO+26, whereas among patients with relapsing GCA, TCZ QW but not TCZ Q2W treatment reduced the risk for flare compared with both PBO groups, and there was separation in the time to flare between the TCZ QW and Q2W groups. The objective of the current analysis was to report time to first flare over 3 years of the GiACTA trial (part 1 plus 2-year open-label part 2) among patients with new-onset or relapsing GCA.
Methods: At the end of part 1, patients entered open-label part 2, in which GCA therapy (including initiation/termination of open-label TCZ and/or glucocorticoids) was given at the investigator’s discretion according to disease status. Time to first GCA disease flare during the 3-year study period was assessed using Kaplan-Meier analysis for patients in the intent-to-treat population according to disease onset status at baseline (new-onset or relapsing) based on their originally assigned treatment groups: TCZ QW, TCZ Q2W, or pooled PBO (PBO+26 and PBO+52).
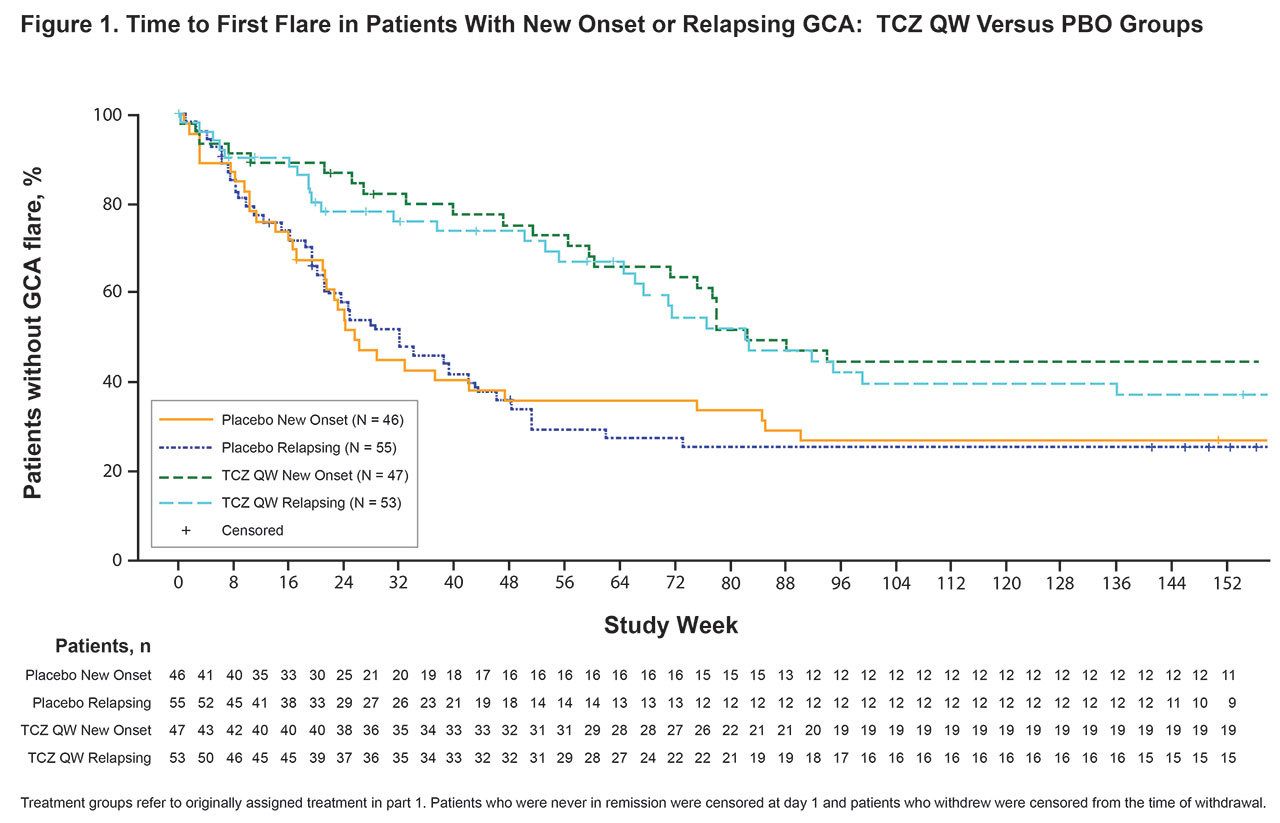
Results: Among patients randomly assigned in part 1, 47 of 100 (47%) in the TCZ QW group, 26 of 49 (53%) in the TCZ Q2W group, and 46 of 101 (46%) in the pooled PBO group had new-onset GCA at baseline; the rest had relapsing GCA. Median time to first flare over 3 years was longer for patients assigned to TCZ treatment in part 1 than for those assigned to PBO. Among patients assigned to TCZ, it was longer with QW than Q2W dosing for both the new-onset and the relapsing subgroups (Table 1). Higher proportions of patients in the TCZ QW group (new-onset, 49%; relapsing, 47%) than the pooled PBO group (new-onset, 28%; relapsing, 31%) and the TCZ Q2W group (new-onset, 27%; relapsing, 35%) remained flare-free during the entire 3-year study. Kaplan-Meier analysis showed a clear separation between the TCZ QW and pooled PBO groups over 3 years for patients with new-onset and relapsing GCA (Figure 1). Separation between the TCZ QW and TCZ Q2W groups was also observed over 3 years in patients with new-onset and relapsing GCA, although this was more evident in patients with new-onset GCA during year 3 (Figure 2).
Conclusion: In this 3-year analysis of GiACTA parts 1 and 2, time to first flare favored TCZ QW over TCZ Q2W in patients with new-onset and relapsing GCA. TCZ QW delayed time to first flare compared with PBO in patients with new-onset and relapsing GCA, supporting TCZ QW dosing in patients with GCA regardless of disease onset.
References: 1. Stone JH et al N Engl J Med 2017;377:317-328.
To cite this abstract in AMA style:
Stone J, Spotswood H, Unizony S, Aringer M, Blockmans D, Brouwer E, Cid M, Dasgupta B, Rech J, Salvarani C, Spiera R, Bao M. Time to Flare in Patients with New-Onset versus Relapsing Giant Cell Arteritis Treated with Tocilizumab or Placebo Plus Prednisone Tapering: 3-Year Results from a Randomized Controlled Phase 3 Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/time-to-flare-in-patients-with-new-onset-versus-relapsing-giant-cell-arteritis-treated-with-tocilizumab-or-placebo-plus-prednisone-tapering-3-year-results-from-a-randomized-controlled-phase-3-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/time-to-flare-in-patients-with-new-onset-versus-relapsing-giant-cell-arteritis-treated-with-tocilizumab-or-placebo-plus-prednisone-tapering-3-year-results-from-a-randomized-controlled-phase-3-trial/