Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The phase 3 KEEPsAKE 1 and KEEPsAKE 2 randomized double-blind clinical trials demonstrate that risankizumab (RZB) provides a high level of durable improvement in musculoskeletal (MSK) as well as dermatological manifestations and has a favorable safety profile in psoriatic disease. This post hoc analysis evaluates the median time to first achievement of clinically meaningful MSK efficacy responses and patient reported outcomes (PROs) in patients with active PsA who are treated with RZB.
Methods: The KEEPsAKE 1 (NCT03675308) and KEEPsAKE 2 (NCT03671148) phase 3 trials enrolled patients ≥ 18 years old with active PsA who responded inadequately or were intolerant to ≥ 1 conventional synthetic DMARD (csDMARD), and patients with previous inadequate response or intolerance to 1 or 2 biological therapies (Bio-IR) and/or ≥ 1 csDMARD, respectively. Patients were randomized at baseline (BL) to receive either RZB 150 mg or placebo (PBO). At week 24, patients receiving PBO were switched to RZB 150 mg (PBO/RZB). This post hoc analysis included the pooled population of bio-naïve patients from the KEEPsAKE 1 and 2 trials who received continuous RZB 150 mg starting at BL. 11 outcomes related to MSK efficacy responses and PROs were evaluated: ≥ 20% improvement in tender joint count (TJC), ≥ 50% improvement in TJC, ≥ 20% improvement in swollen joint count (SJC), ≥ 50% improvement in SJC, minimum clinically important difference (MCID) in pain (≥ 10 point decrease on the 0-100 visual analogue scale, VAS), 30% improvement in pain, MCID in FACIT – Fatigue (≥ 4 point increase), MCID in HAQ-DI (≥ .35 point decrease), MCID in Patient Global Assessment of Disease Activity, PtGA (≥ 10 point decrease), MCID in SF-36 mental component summary, SF-36 MCS (≥ 2.5 point increase), MCID in SF-36 physical component summary, SF-36 PCS (≥ 2.5 point increase). The median and 25th percentiles of time to first achievement of efficacy outcomes were calculated using Kaplan-Meier estimates at week 52; patients who did not achieve the outcomes by week 52 were censored at their last visit by the cutoff date.
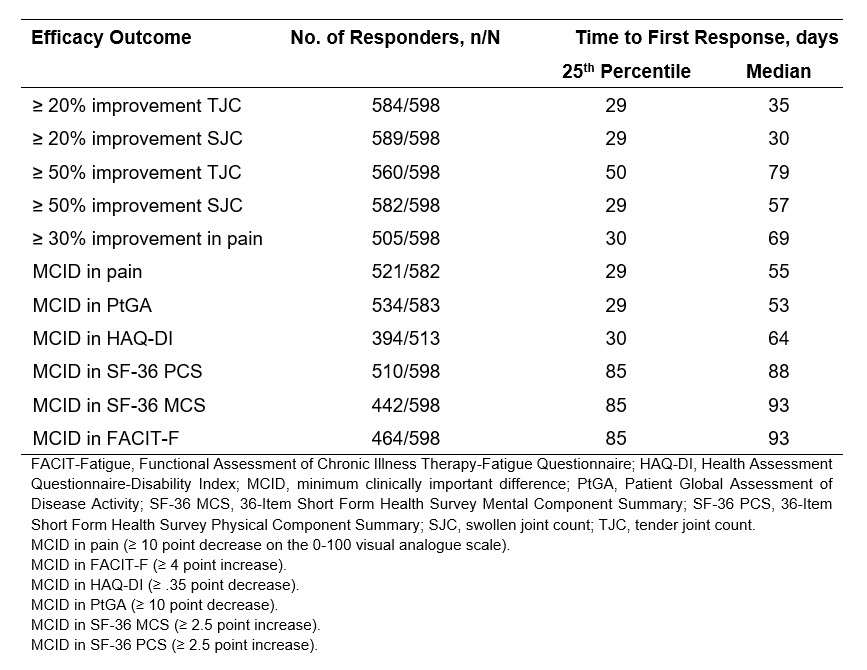
Results: A total of 598 bio-naïve patients from the KEEPsAKE 1 and 2 trials were included in the pooled analysis, with a high number of responders across the assessed outcomes (Table 1). The median time to first achieve ≥ 20% improvement in TJC and SJC was 35 and 30 days, respectively. Median time to first achieve ≥ 50% improvement in TJC and SJC was 79 and 57 days, respectively. Median time to first achieve ≥ 30% improvement in pain was 69 days and to first achieve MCID in pain was 55 days. Median time to first achieve MCID in PtGA, HAQ-DI, SF-36 PCS, SF-36 MCS, and FACIT-F was 53, 64, 88, 93, and 93 days, respectively (Table 1). For patients who first achieved 20% improvement in TJC/SJC, and MCID in HAQ-DI/pain by the median time taken for each initial response, BL characteristics were consistent with the overall bio-naïve population.
Conclusion: This post hoc analysis of the median time to first response in key MSK responses and PROs demonstrates early median time to clinically meaningful efficacy for patients with PsA treated with RZB, with related improvement in quality of life.
To cite this abstract in AMA style:
Tillett W, Rednic S, Mizelle K, Ritchlin C, Khattri S, Shi L, Bialik B, Iyile T, Kavanaugh A. Time to First Clinically Meaningful Efficacy Responses in Musculoskeletal and Patient Reported Outcomes in Patients with Active Psoriatic Arthritis Treated with Risankizumab: A Post Hoc Analysis of the Phase 3 KEEPsAKE 1 and KEEPsAKE 2 Trials [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/time-to-first-clinically-meaningful-efficacy-responses-in-musculoskeletal-and-patient-reported-outcomes-in-patients-with-active-psoriatic-arthritis-treated-with-risankizumab-a-post-hoc-analysis-of-th/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/time-to-first-clinically-meaningful-efficacy-responses-in-musculoskeletal-and-patient-reported-outcomes-in-patients-with-active-psoriatic-arthritis-treated-with-risankizumab-a-post-hoc-analysis-of-th/