Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriatic arthritis (PsA) is an inflammatory disease that can manifest with considerable clinical heterogeneity. Secukinumab demonstrated improvements across the updated Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and Outcome Measures in Rheumatology (GRAPPA-OMERACT) PsA core domains in a pooled population of patients with PsA.1 However, the relative time to response across disease domains has not been studied. The objective of this post hoc analysis was to evaluate time to achievement of clinical responses across the GRAPPA-OMERACT core domains in patients with PsA treated with secukinumab in the FUTURE 2-5 studies.
Methods: This post hoc analysis evaluated data pooled from 1366 patients with PsA who were randomized at baseline to receive secukinumab 150 mg or 300 mg in the phase 3 FUTURE 2-5 studies (NCT01752634, NCT01989468, NCT02294227, and NCT02404350). Efficacy outcomes assessed across GRAPPA-OMERACT PsA core domains included musculoskeletal (MSK) disease activity, skin and nail disease activity, systemic inflammation, pain, health-related quality of life (HRQOL), fatigue, and physical function. For each outcome, the proportion of patients achieving minimal clinically important difference (MCID) or complete resolution was assessed through Week 52 using as-observed data. Median time to the initial efficacy response and the response rate by Week 52 were estimated using the Kaplan-Meier method.
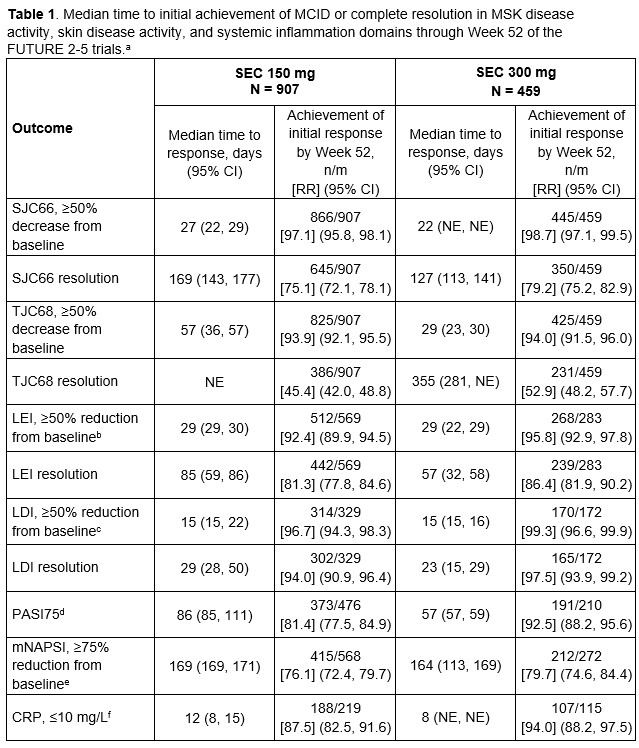
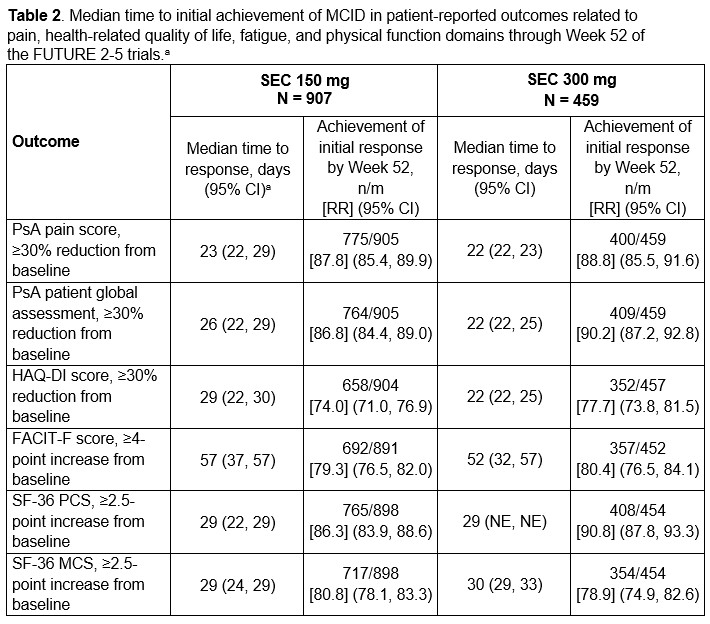
Results: Patients treated with either dose of secukinumab generally experienced rapid improvements across GRAPPA-OMERACT domains. Median time to achievement of MCID in the systemic inflammation domain was < 2 weeks, followed by improvements across MSK disease activity outcomes generally within the first 4 weeks of secukinumab treatment (Table 1). Patients with dactylitis at baseline achieved resolution of dactylitis within approximately 4 weeks, while the median time to resolution of enthesitis in those with enthesitis at baseline was approximately 12 weeks (Table 1). Among patients with psoriasis at baseline, the median time to achievement of 75% reduction in the Psoriasis Area Severity Index score (PASI75) was approximately 8 to 12 weeks; patients with nail psoriasis at baseline achieved 75% improvement in the modified Nail Psoriasis Severity Index score (mNAPSI) after approximately 24 weeks of treatment (Table 1). Patients experienced rapid improvements in pain, HRQOL, and physical function domains (Table 2), consistent with the timing of improvements in MSK symptoms. Median achievement of MCID in the fatigue domain occurred slightly later, after approximately 7 to 8 weeks of secukinumab treatment (Table 2).
Conclusion: Patients with PsA receiving secukinumab 150 mg or 300 mg in the FUTURE 2-5 trials generally experienced rapid improvements across the GRAPPA-OMERACT core domains with patients achieving MCID by approximately 4 weeks of treatment. Improvements in MSK symptoms coincided with improvements in pain, HRQOL, and physical function, which occurred earlier than skin and nail psoriasis improvements. The interdependence of distinct domain response sequencing requires further study.
Reference
1. Orbai A-M, et al. Ann Rheum Dis 2017;76:673-680.
a Median time, RR and associated 95% CI are from the Kaplan-Meier estimate taking censoring into consideration.
b Among patients with enthesitis at baseline.
c Among patients with dactylitis at baseline.
d Among patients with psoriasis at baseline.
e Among patients with nail psoriasis at baseline.
f Among patients with CRP >10 mg/L at baseline.
a Median time, RR and associated 95% CI are from the Kaplan-Meier estimate taking censoring into consideration.
To cite this abstract in AMA style:
Coates L, McInnes I, Husni M, Vizcaya C, Bao W, Mease P. Time to Clinical Response to Secukinumab Across Disease Domains Among Patients with Psoriatic Arthritis: A Pooled Post Hoc Analysis of 4 Phase 3 Trials [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/time-to-clinical-response-to-secukinumab-across-disease-domains-among-patients-with-psoriatic-arthritis-a-pooled-post-hoc-analysis-of-4-phase-3-trials/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/time-to-clinical-response-to-secukinumab-across-disease-domains-among-patients-with-psoriatic-arthritis-a-pooled-post-hoc-analysis-of-4-phase-3-trials/