Session Information
Date: Sunday, November 5, 2017
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Baricitinib (BARI), an oral, selective Janus kinase (JAK)1/2 inhibitor1, has shown efficacy in DMARD naïve RA patients (pts)2 and in pts with inadequate response to methotrexate (MTX-IR).3 In the EU, BARI is approved for treating moderate to severe active RA in adults. The objective of this analysis is to evaluate the time to achieve moderate disease activity (MDA), low disease activity (LDA), and remission in pts treated with BARI 4-mg compared to placebo (PBO) or active comparators, MTX and adalimumab (ADA) from the RA-BEGIN2 and RA-BEAM3 trials.
Methods: In RA-BEGIN, DMARD naïve pts were randomized to BARI 4-mg once daily (QD), MTX or BARI 4-mg+MTX for 52 weeks (wks). In RA-BEAM, MTX-IR pts were randomized to PBO, BARI 4-mg QD or ADA 40-mg biweekly for 52 wks (PBO switched to BARI 4-mg at 24 wks). This post hoc analysis estimated the time to achieve MDA (Clinical Disease Activity Index, CDAI ≤22), LDA (CDAI ≤10), and remission (CDAI ≤2.8) in modified intent-to-treat (mITT) pts and in the subset of pts with high baseline disease activity (HDA) defined as CDAI >22 from the two trials. Cumulative incidence of MDA, LDA, and remission over 52 wks for RA-BEGIN and 24 wks for RA-BEAM were estimated.4 Hazard ratios between treatments were obtained using Cox proportional hazards regression adjusting for region and baseline joint erosions (1-2 or ≥3 erosions) without control for multiple comparisons.
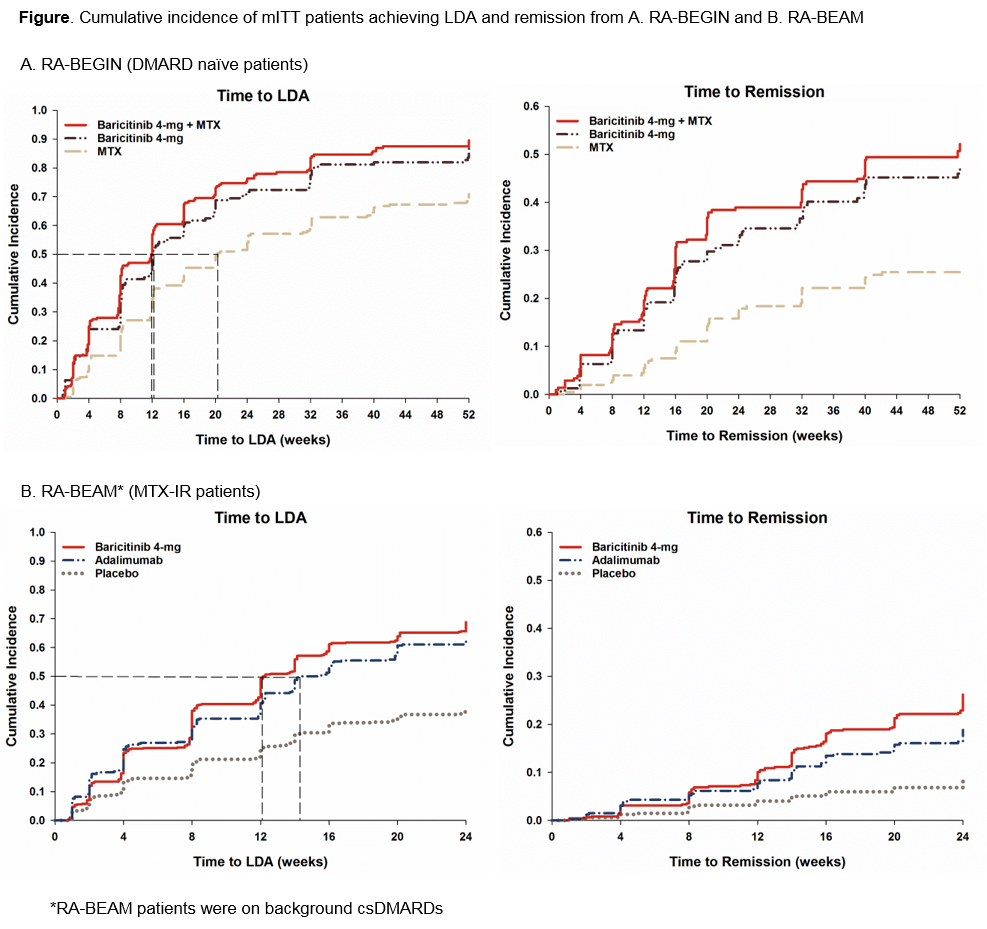
Results: In DMARD naïve population, BARI 4-mg monotherapy pts were 1.6 times more likely to achieve MDA and LDA and twice more likely to achieve remission compared to MTX (p<0.001) (Table). Median time to MDA and LDA with BARI 4-mg treatment (2 and 12 wks, respectively) was 2 and 8 wks shorter than with MTX (4 and 20 wks, respectively) (Figure). Bari 4-mg+MTX performed similar to BARI 4-mg monotherapy.
In MTX-IR population, BARI 4-mg treated pts were 1.7, 2.3, and 3.5 times more likely to achieve MDA, LDA, and remission than PBO (p<0.001) and 1.0, 1.1, 1.4 times more likely to achieve MDA (p=0.557), LDA (p=0.295), and remission (p=0.030) than ADA (Table). Median time to MDA was 5 wks shorter with BARI 4-mg treatment (2 wks) than PBO (7 wks) but similar to ADA (2 wks). Median time to LDA with BARI 4-mg (12 wks) was 2 wks shorter than ADA (14 wks) while PBO pts never reached the median time to LDA during the 24 wk study (Figure). Consistent results were obtained in pts with HDA.
Conclusion: DMARD naïve and MTX-IR pts were more likely to achieve LDA and remission, and at a faster pace, with BARI 4-mg than the comparator treatment groups.
References: 1. Fridman JS J Immunol 2010 2. Fleischmann R Arthritis Rheumatol 2017 3. Taylor PC N Engl J Med 2017 4. Gooley TA Stat Med 1999
To cite this abstract in AMA style:
Keystone EC, Dougados M, Ruderman EM, Zhu B, Lopez-Romero P, Lund H, Cardoso A, Schlichting DE, Martinez Osuna P, Ortmann R, Kvien TK. Time to Achieve Moderate/Low Disease Activity and Remission in RA Patients on Baricitinib Compared to Adalimumab, Methotrexate, and Placebo [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/time-to-achieve-moderatelow-disease-activity-and-remission-in-ra-patients-on-baricitinib-compared-to-adalimumab-methotrexate-and-placebo/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/time-to-achieve-moderatelow-disease-activity-and-remission-in-ra-patients-on-baricitinib-compared-to-adalimumab-methotrexate-and-placebo/