Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Lupus Therapeutics (LT), the clinical affiliate of the Lupus Research Alliance, oversees the Lupus Clinical Investigators Network (LuCIN), a network of premier research sites in North America formed to accelerate and improve the conduct of clinical trials for the development of new therapies. An annual survey of LuCIN investigators and research teams aims to define and assess challenges and solutions to conducting lupus clinical trials.
Methods: The 2024 LuCIN annual survey collected data from January 7 to February 18, 2024. Questions focused on assessing views of challenges and solutions for conducting lupus clinical trials in North America. Descriptive statistics were used to analyze the survey responses.
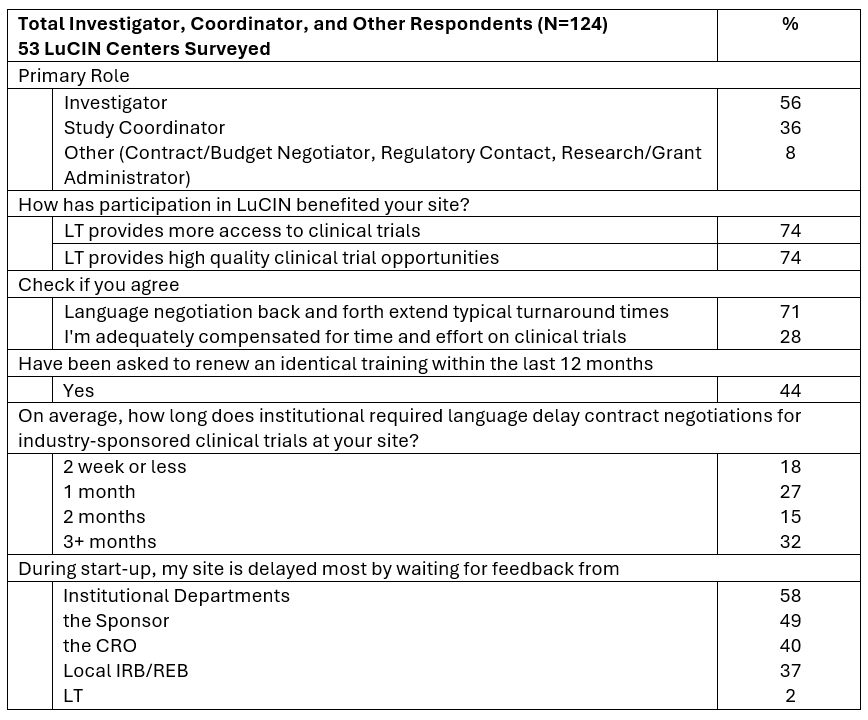
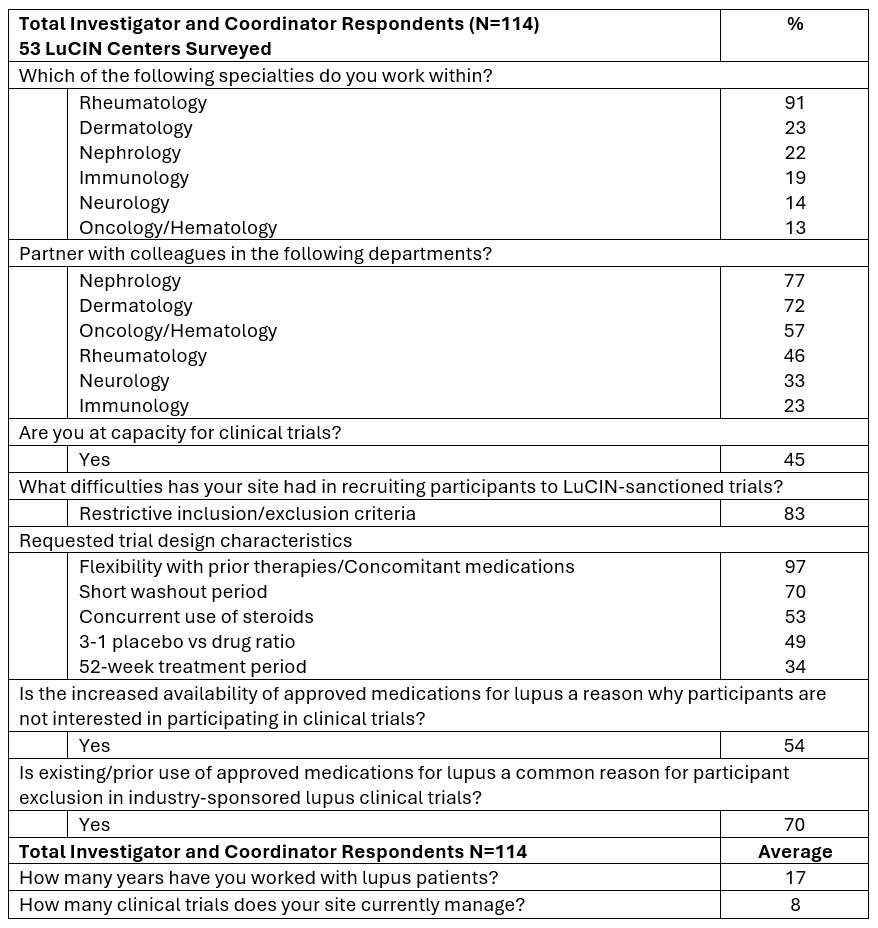
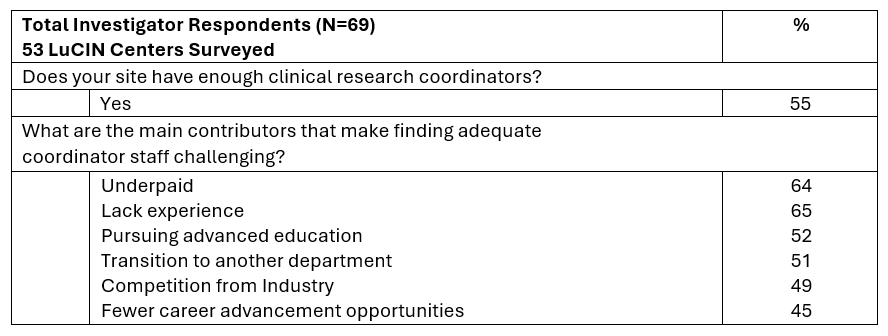
Results: 124 lupus clinical trial site staff surveyed Figure 1, revealed 74% appreciate increased access to sponsored clinical trials and high-quality clinical trial opportunities provided by LT. Still, only 28% of respondents feel adequately compensated for time and effort on clinical trials and 44% have renewed identical training for sponsors within the last 12 months. Contracting negotiations are often delayed by institutional required language, affecting most respondents for 1-3+ months Figure 1. Feedback delays during start-up activities are mostly from Institutional Departments (58%) and the Sponsor (49%), notably LT (2%) and others Figure 1. 114 investigators and coordinators work mostly within Rheumatology (91%) and Dermatology (23%), collaborate most frequently with Nephrology (77%), Dermatology (72%), and Oncology/Hematology (57%). Respondents had an average of 17 years of experience with lupus patients, managing 8 clinical trials on average and almost half (45%) agreed they were at capacity for clinical trials Figure 2. Survey respondents describe restrictive trial eligibility criteria (83%) as a major recruitment challenge and outlined design preferences Figure 2. Over half (54%) agreed that the availability of approved medications deters participant clinical trial interest, and 70% say prior use of treatments for lupus leads to participant exclusion. Nearly half of the 69 investigators (45%) report a shortage of research coordinators to enable study participation due to various reasons Figure 3.
Conclusion: Feedback from lupus trial centers in North America underscores the need to address challenges in clinical trial conduct and design to enable better support for site teams, especially research coordinators. The responses indicate an appreciation for the enhanced access to high quality clinical trials afforded by being part of a structured clinical trials network. Further efforts are needed to streamline processes, provide better support for site staff, and establish easy methods to accept institutional required language and prior training. Further research should address interdepartmental collaborations given the growing cell therapy landscape, alongside evaluating flexible trial designs to improve recruitment and manage a high workload effectively. These insights allow for better support and function of clinical network centers that ultimately benefit those impacted by lupus by accelerating lupus trials and treatment development.
To cite this abstract in AMA style:
Jackson B, Dall'Era M, Sheikh S, Zhang X, Irons T, Meriwether J, Merrell M, Bell S. Through the LuCIN Lens: Defining Barriers and Forging Solutions for Lupus Clinical Trials in North America [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/through-the-lucin-lens-defining-barriers-and-forging-solutions-for-lupus-clinical-trials-in-north-america/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/through-the-lucin-lens-defining-barriers-and-forging-solutions-for-lupus-clinical-trials-in-north-america/