Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: RA patients typically present with pain and morning stiffness (MS) in addition to joint swelling and tenderness. Nocturnal inflammatory cytokines are assumed to be associated with MS. DR prednisone given at bedtime has previously shown significant absolute and relative reduction in MS and early morning cytokines compared to both conventional (IR) prednisone and placebo in well controlled studies.(1-3) Studies of categorical and comparative MS responses to DR and IR prednisone are lacking. In this new analysis of Phase 3 study data, we completed a time-to-event analysis of morning stiffness.
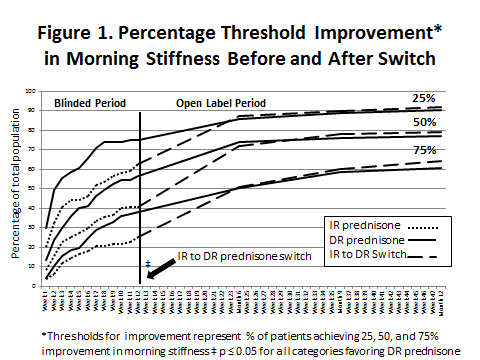
Methods: CAPRA-1, a 12-week, double-blind, controlled study, randomized patients to IR-prednisone taken in the morning or DR-prednisone taken once daily at bedtime (eg, 10pm) in addition to standard DMARD treatment.(1) In the following open label phase, patients previously receiving IR prednisone (N=110) were then switched to DR-prednisone and followed at 3, 6, and 9 months.(2) Patients previously on DR-prednisone (N=97) continued on therapy. Patients completed diary entries at least 7 days prior to each visit including waking time, stiffness (yes/no), time of resolution and intensity of pain (VAS) during the day. In a novel analysis, all diary entries +/- 4 weeks of each scheduled visit were analyzed over 1 year for threshold response (see Figure 1). Weekly data was available during the DB study. Chi-square tests for category treatment differences were completed at end of DB and months 6, 9, and 12. Kaplan-Meier estimates and p-values were computed using a Cox proportional model. The response categories were not mutually exclusive.
Results: The DR prednisone arm had statistically significantly more responders in each of the 3 MS response categories at the end of the DB period as compared to IR prednisone (p≤ 0.05). DR prednisone had higher percentages of responders in the 3 response categories at each 12 week evaluation. The separation started after 1 week of therapy. Of those who had responses in the DB study, it took longer for the IR prednisone group to reach the same level of response. The IR prednisone group, when switched to DR prednisone, had comparable responses in all categories within 3 months and significantly shorter time to response when compared to patients already receiving DR prednisone (p ≤ 0.008).
Conclusion: DR prednisone, as compared to IR prednisone, produces significantly higher MS response rates as defined by 25/50/75% improvement thresholds. The time to reach these thresholds is quicker with DR prednisone and patients switched to DR prednisone from IR prednisone quickly “catch up” and manifest comparable responses. Patients treated with DR prednisone maintained their response with no evidence of tachyphylaxis up to 1 year.
References
(1) Buttgereit, et. al. Lancet 2008;371:205-214.
(2) Buttgereit, et. al. Ann Rheum Dis 2010;69:127-1280.
(3) Buttgereit, et. al. Ann Rheum Dis 2013;72:204–210
Disclosure:
F. Buttgereit,
Horizon Pharma, Inc,
2;
J. Kent,
Horizon Pharma, Inc,
3;
R. Holt,
Horizon Pharma, Inc,
5;
A. Grahn,
Horizon Pharma, Inc,
3;
P. Rice,
Horizon Pharma, Inc,
5;
R. Alten,
Horizon Pharma, Inc,
5;
Y. Yazici,
Horizon Pharma, Inc,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/threshold-analysis-of-patient-reported-morning-stiffness-where-delayed-release-dr-prednisone-was-compared-to-and-replaced-immediate-release-prednisone-in-rheumatoid-arthritis-ra-patients-receivi/