Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Hydroxychloroquine (HCQ) is the cornerstone of lupus (or SLE) treatment. Yet the optimal dosing of HCQ in SLE is unknown. Reducing HCQ dose to 5 mg/kg to limit toxicity per American Academy of Ophthalmologists (AAO) guidelines, increases lupus flare risk by 6-fold. Therefore, establishing effective reference ranges of HCQ blood levels with upper and lower bounds for efficacy may inform individualizing HCQ dosing to maximize efficacy and limit toxicity. While studies suggest various thresholds for therapeutic HCQ blood levels, none have offered a therapeutic range with upper and lower bounds for HCQ levels that predict minimum and maximum efficacy. Moreover, clinical interpretation of a threshold is difficult as it may not account for changes in patient-level factors such as weight and kidney function and fluctuations in HCQ blood levels over time. Thus, the objectives of this study were to: 1) examine the association between HCQ blood levels and high lupus disease activity (HDA) in a prospective SLE cohort; 2) define therapeutic thresholds of HCQ blood levels with upper and lower bounds for HCQ blood levels that predict maximum and minimum efficacy.
Methods: This study measured HCQ blood levels in unique SLE visits using liquid chromatography-tandem mass spectrometry. HCQ blood levels and SLE disease activity index (SLEDAI) scores were measured on the day of the visit for each patient. High lupus disease activity (HDA) was defined as SLEDAI scores of ≥6. We performed nonparametric spline regression analysis to identify HCQ blood levels cut-points associated with reduction in the odds of HDA. To identify an effective therapeutic range with lower and upper limits of HCQ blood levels that are associated with lower odds of HDA, we examined associations between HDA and every 50-100 ng/ml increase in HCQ blood levels starting at 100 ng/ml through 1500 ng/ml using univariable and multivariable logistic regression models. Factors that can affect HCQ levels, such as kidney function, HCQ dose and timing, were included in analyses.
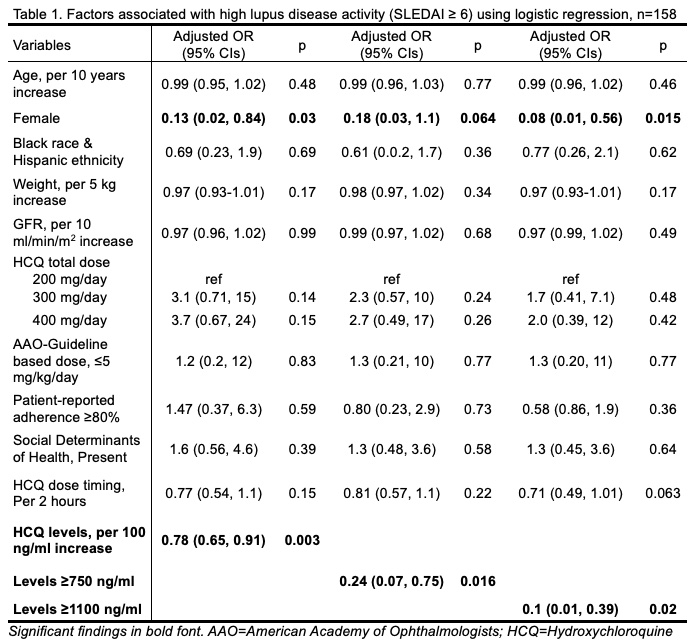
Results: Among 158 SLE patients in whom HCQ blood levels were measured, 92% were women and 32% were of Black race or Hispanic ethnicity. HDA was noted in 18% of patients.
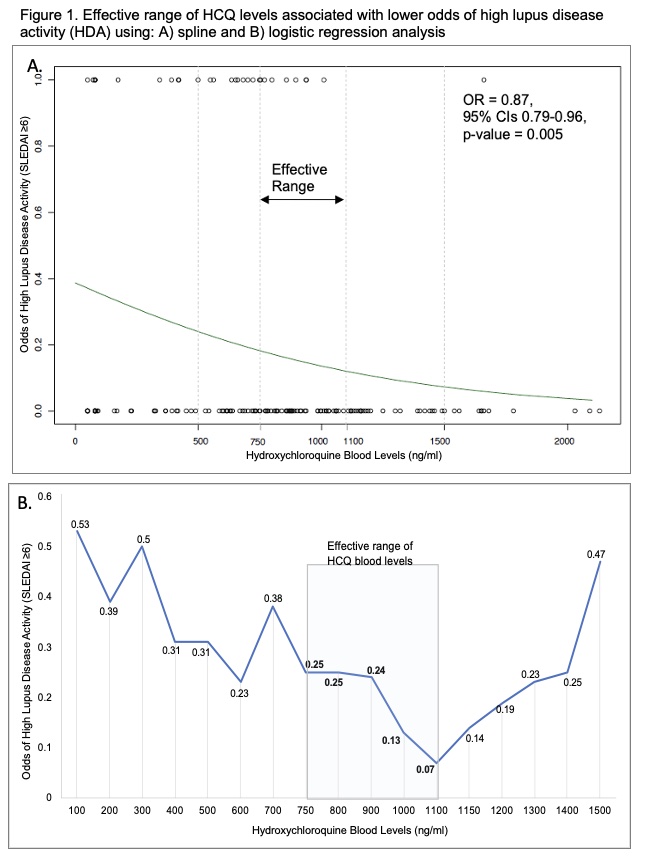
Using nonparametric spline regression models, we noted that HCQ blood levels, 750 and 1100 ng/ml, significantly reduced the odds of HDA (Figure 1A). HCQ blood levels < 700 & >1150 did not reduce the odds of HDA (Figure 1B & Table 1). Levels of 750 ng/ml lowered the lower odds of HDA by 76% (OR 0.24, p=0.016, Fig 1B & Table 1). A sustained reduction in the odds of HDA with HCQ blood levels was noted through 1000 ng/ml. Peak effects were noted at levels of 1100 ng/ml with a 90% reduction in the odds of HDA (Figure 1B. & Table 1). Figure 2. illustrates how these findings could guide clinicians to individualize HCQ dosing to achieve therapeutic blood levels to maximize efficacy, while balancing safety.
Conclusion: An effective therapeutic range of HCQ levels, 750-1100 ng/ml, significantly reduced the odds of high lupus disease activity by 76-90%. These findings could be guide clinicians to individualize HCQ dosing to maximize efficacy during routine lupus visits.
activity (HDA) using: A) spline and B) logistic regression analysis
To cite this abstract in AMA style:
Garg S, Chewning B, Astor B, Bartels C. Therapeutic Range of Hydroxychloroquine Blood Levels May Reduce Odds of High Lupus Disease Activity [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/therapeutic-range-of-hydroxychloroquine-blood-levels-may-reduce-odds-of-high-lupus-disease-activity/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/therapeutic-range-of-hydroxychloroquine-blood-levels-may-reduce-odds-of-high-lupus-disease-activity/