Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Low trough levels of the tumor necrosis factor inhibitor, adalimumab (ADL), and anti-ADL antibodies (AAA) were reported to be correlated with lack of response at later time points in patients (pts) with rheumatoid arthritis (RA).1
Objective: To assess the ability of ADL trough levels and clinical assessments at Week 12 to predict clinical remission (REM) after 24 weeks (wks) of treatment with ADL +/- methotrexate (MTX) in established RA pts.
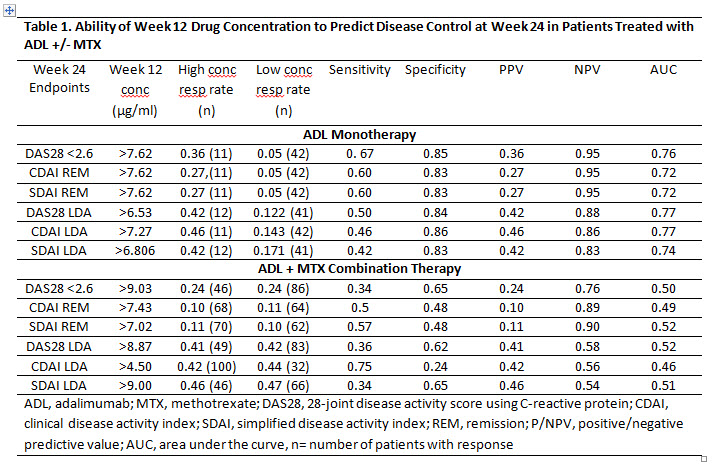
Methods: Data from MTX inadequate responders (MTX-IR) pts with established RA with available measurement of ADL trough levels and clinical assessments at Wks 12 and 24 from several clinical trials were pooled: for pts who received ADL+MTX combination therapy from DE009, DE019, M10-261 and M13-390; for pts who received ADL monotherapy from DE011, M10-261 and M13-390. Efficacy endpoints at Wk 24 were DAS28-CRP <2.6 and DAS28-CRP low disease activity (LDA, <3.2), remission (REM) and LDA by simplified disease activity index (SDAI, ≤3.3 and ≤11 respectively); REM and LDA by clinical disease activity index (CDAI, ≤2.8 and ≤10 respectively). Each of the pooled datasets was randomly and equally split into training and testing sets. Predictive modeling was performed on the training set, and the best-performing model was selected and validated in the testing set. The performance of the final model was reported based on the testing set.
Results: Based on the cutoffs selected by the predictive model, ADL concentrations at Wk 12 were only slightly predictive for Wk 24 clinical assessment in the ADL monotherapy group, but not in the ADL+MTX group (Table 1). However, based on achievement of the specified CDAI, SDAI or DAS28-CRP score at Wk 12 (selected by the model), pts were correctly predicted to reach Wk 24 REM or LDA with an accuracy of 80-90% and area under the receiver operating characteristic curve (AUC) of 75-90% (Table 2). As an example, pts on ADL monotherapy with DAS28 < 3.3 at Wk 12 had 60% and 70% chance of reaching Wk 24 DAS28-CRP<2.6 and LDA respectively, whereas pts with DAS28 ≥ 3.3 had 0% and 7% chance of achieving Wk 24 DAS28-CRP<2.6 and LDA, respectively (Table 2). Pts on ADL+MTX with Wk 12 SDAI <12.5 had a 25% and 77% chance of achieving SDAI REM and LDA at Wk 24, respectively.
Conclusion: The ADL concentrations at Week 12 selected by the prediction model were weak predictors of disease control at 6 months, especially for pts on ADL+MTX combination therapy. However, using the model-selected cutoffs of composite clinical endpoints at Wk 12, disease control after 6 months of ADL +/- MTX treatment could be correctly predicted in 70-80% of pts.
To cite this abstract in AMA style:
Smolen JS, Mostafa N, Huang X, Noertersheuser P, Klünder B, Chen K, Kalabic J, Sainsbury I, Oerlemans R, Florentinus S, Burmester GR. The Value of Adalimumab Trough Levels and Clinical Assessments in Predicting Clinical Response in Patients with Established Rheumatoid Arthritis and an Inadequate Response to Methotrexate [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/the-value-of-adalimumab-trough-levels-and-clinical-assessments-in-predicting-clinical-response-in-patients-with-established-rheumatoid-arthritis-and-an-inadequate-response-to-methotrexate/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-value-of-adalimumab-trough-levels-and-clinical-assessments-in-predicting-clinical-response-in-patients-with-established-rheumatoid-arthritis-and-an-inadequate-response-to-methotrexate/