Session Information
Session Type: Abstract Submissions
Session Time: 5:15PM-5:45PM
Background/Purpose: Variants in the SLCO1B1 gene, encoding a hepatic methotrexate (MTX) transporter, affect clearance of high-dose MTX in leukemia patients. We aimed to assess the influence of this gene on the response to MTX in juvenile idiopathic arthritis (JIA) patients.
Methods: 314 JIA patients treated with methotrexate monotherapy enrolled in the local clinic over more than 30 years or in multi-center gene expression studies comprised the initial cohort. Active joint counts at baseline and follow up (visit closest to six months of treatment) were available for all patients and used to evaluate response. Patients were excluded if they had less than two active joints (n=3) at the baseline visit, had Down Syndrome (n=3), if their subtype was ERA (n=3) or systemic (n=8). Data on the route of administration of MTX was available on 174 patients. Genotyping of 3 SNPs in SLCO1B1 (rs4149056, rs2306283 and rs11045819) was performed successfully on 286 patients with TaqMan probes or available from genome-wide arrays. A patient’s SLCO1B1 diplotype was determined by these three SNPs that make up the *1a, *1b, *4, *5, *14 and *15 alleles. Percent change in active joint count at follow-up was used as the dependent variable in a linear regression.
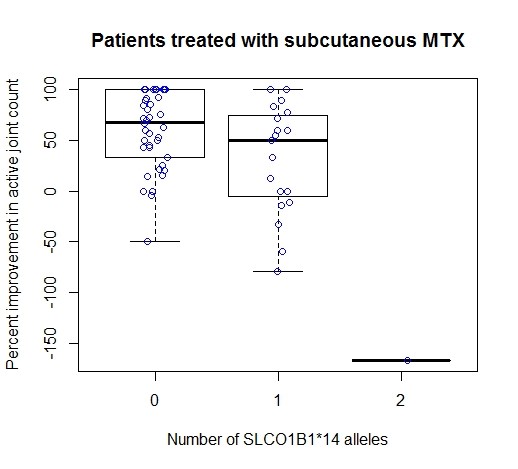
Results: In a univariate analysis, the SLCO1B1 *14 allele was associated with less response to MTX (p=0.048). In univariate analyses for other variables, the route of administration of MTX and sex were marginally associated with response (better response with subcutaneous MTX and in females, p=0.07 for each). Variables tested but not associated with response to MTX were: number of active joints at baseline, MTX dose at baseline, follow-up time, age, race, and JIA subtype. In a multivariate model including sex (p=0.04), route (p=0.06) and number of *14 alleles (p=0.09), 4% of the variability in response was explained. Among the 286 patients included in the study, there were 62 patients treated with subcutaneous MTX. The *14 allele explains 18.7% of the variability in response (p=0.00026) in the patients where MTX was administered subcutaneously (Figure) but is not associated with response in the 112 patients treated with oral MTX.
Conclusion: The SLCO1B1*14 allele may be associated with poor response to subcutaneous MTX for JIA patients. This allele has been associated with fast clearance and low exposure after high dose MTX in leukemia patients. Thus, the SLCO1B1 *14 allele may be informative for precision dosing of MTX in JIA patients. Patients carrying this allele may require a higher dose than non-carriers to achieve a similar response to subcutaneous MTX.
To cite this abstract in AMA style:
Moncrieffe H, Ramsey LB, Sudman M, Gottlieb B, Langefeld CD, Lovell D, Thompson SD. The SLCO1B1 *14 Allele is Associated with Poor Response to Subcutaneous Methotrexate in Patients with Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 4). https://acrabstracts.org/abstract/the-slco1b1-14-allele-is-associated-with-poor-response-to-subcutaneous-methotrexate-in-patients-with-juvenile-idiopathic-arthritis/. Accessed .« Back to 2017 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-slco1b1-14-allele-is-associated-with-poor-response-to-subcutaneous-methotrexate-in-patients-with-juvenile-idiopathic-arthritis/