Session Information
Date: Sunday, November 5, 2017
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Upadacitinib is a selective JAK1 inhibitor being developed for the treatment of several inflammatory diseases, including rheumatoid arthritis (RA). Upadacitinib showed favorable efficacy and acceptable safety profiles in two Phase 2 studies in subjects with RA. Currently, doses of 15 mg and 30 mg once daily (QD) are being evaluated in ongoing Phase 3 studies in RA. Oral contraceptives are expected to be commonly used with upadacitinib in patients with inflammatory diseases. This study evaluated the effect of multiple doses of upadacitinib on pharmacokinetics of ethinylestradiol and levonorgestrel.
Methods:
Healthy female subjects (N = 20) received single doses of a combined oral contraceptive tablet containing 30 μg ethinylestradiol and 150 μg levonorgestrel alone (Study Period 1) and on Day 12 of a 14-day regimen of upadacitinib 30 mg QD (Study Period 2). Upadacitinib was administered in the study using the extended-release tablet formulation being utilized in Phase 3 studies in RA. Blood samples for ethinylestradiol and levonorgestrel assays were collected by venipuncture prior to and for 96 hours after the oral contraceptive administration in each Study Period. Pharmacokinetic parameters for ethinylestradiol and levonorgestrel were calculated using non-compartmental analyses.
Results:
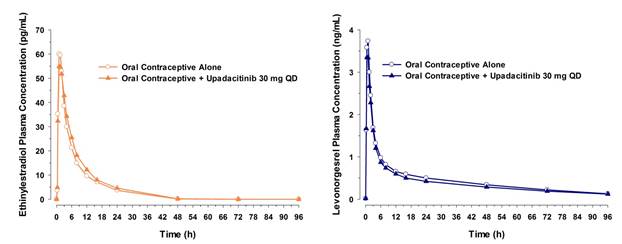
The ratios (90% confidence intervals) for Cmax and AUCinf following administration of the oral contraceptive with upadacitinib compared with administration of the oral contraceptive alone were 0.96 (0.89 to 1.02) and 1.11 (1.03 to 1.19), respectively, for ethinylestradiol and 0.96 (0.87 to 1.06) and 0.95 (0.85 to 1.07), respectively, for levonorgestrel.
Figure 1. Plasma Concentration versus Time Profiles for Ethinylestradiol and Levonorgestrel Following Administration of the Combined Oral Contraceptive Alone and with Upadacitinib 30 mg QD
Conclusion:
Upadacitinib has no effect on pharmacokinetics of ethinylestradiol and levonorgestrel; the 90% confidence intervals for the ratios of ethinylestradiol and levonorgestrel AUC and Cmax when administered with upadacitinib relative to when administered alone were within the bioequivalence boundaries of 0.8 to 1.25. Therefore, oral contraceptives containing ethinylestradiol or levonorgestrel can be concomitantly administered with upadacitinib.
To cite this abstract in AMA style:
Mohamed MEF, Trueman S, Feng T, Friedman A, Othman AA. The Selective JAK1 Inhibitor Upadacitinib Has No Effect on Pharmacokinetics of the Hormonal Contraceptives Levonorgestrel and Ethinylestradiol [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/the-selective-jak1-inhibitor-upadacitinib-has-no-effect-on-pharmacokinetics-of-the-hormonal-contraceptives-levonorgestrel-and-ethinylestradiol/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-selective-jak1-inhibitor-upadacitinib-has-no-effect-on-pharmacokinetics-of-the-hormonal-contraceptives-levonorgestrel-and-ethinylestradiol/