Session Information
Date: Sunday, November 13, 2016
Title: Metabolic and Crystal Arthropathies - Poster I: Clinical Practice
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Benzbromarone is a potent uricosuric, but is not widely available due to concerns about hepatotoxicity. In Aotearoa New Zealand benzbromarone has been available since April 2013, subject to funding restrictions, for patients with inadequate urate-lowering response or intolerance to allopurinol and probenecid. The aim of this study was to assess the safety and efficacy of benzbromarone in a real-life setting.
Methods: All patients who received funding for benzbromarone from 1/4/2013 to 30/9/2014 were identified. Prescribers were sent a questionnaire for each individual. Information on demographics, efficacy of previous urate-lowering drugs and reasons for discontinuation were collected. Specific information about the dose, effect on serum urate, adverse effects and liver function tests after commencing benzbromarone was recorded.
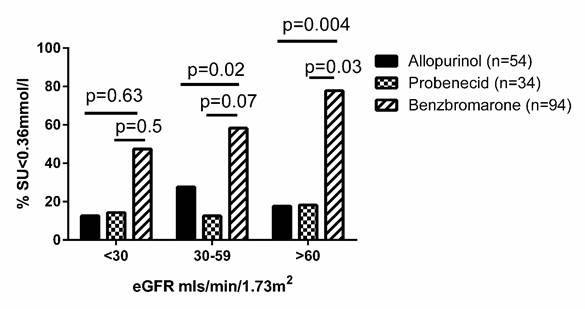
Results: Completed questionnaires were returned for 123/164 (75%) patients. 85 (69.1%) were male, 70 (56.9%) were New Zealand European and 46 (37.4%) were Māori or Pacific Island. The mean (SD) duration of gout was 15 (9.4) years and tophi were present in 70 (56.9%). Mean (SD) SU prior to any ULT was 0.61 (0.11) mmol/l (range 0.36-0.94 mmol/l; n=106). Mean (SD) serum urate prior to benzbromarone was 0.57 (0.12) mmol/l and estimated glomerular filtration rate (eGFR) 50.3 (22.8) ml/min/1.73m2. The median dose of benzbromarone was 100mg/day (25-200mg/day). Six months after commencing benzbromarone, mean (SD) serum urate was 0.35 (0.12) mmol/l. Renal impairment was common, with 22/112 (19.6%) patients having eGFR <30mls/min/1.72m2, 55/112 (49.1%) eGFR >30-59 mls/min/1.72m2 and 35/112 (31.3%) eGFR >60mls/min/1.72m2. With each drug there was no statistically significant difference in the number of patients who achieved SU<0.36mmol/l based on eGFR. However, at each eGFR numerically more people on benzbromarone achieved SU<0.36 mmol/l, compared with the other two allopurinol and probenecid (Figure 1).
Benzbromarone related adverse events included rash (n=4), diarrhoea (n=9), nausea (n=6), and urate stones (n=3). Liver function tests abnormalities were uncommon and tended to be mild. There were 14 patient deaths; none were considered related to benzbromarone. Allopurinol had been prescribed prior to benzbromarone in 117/123 patients; median maximum allopurinol dose was 200mg/day (range 25-600mg/day), and 19% patients received allopurinol >300mg/day.
Conclusion: Benzbromarone provides useful urate-lowering efficacy and does not appear unsafe in patients with gout. It remains effective even in those with renal impairment. Urate-lowering therapy prescribing requires further optimisation.
To cite this abstract in AMA style:
Stamp LK, Haslett J, Frampton C, White D, Gardner D, Stebbings S, Taylor G, Grainger R, Kumar R, Kumar S, Kain T, Porter D, Corkill M, Cathro A, Metcalfe S, Wyeth J, Dalbeth N. The Safety and Efficacy of Benzbromarone in Gout in Aotearoa New Zealand [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/the-safety-and-efficacy-of-benzbromarone-in-gout-in-aotearoa-new-zealand/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-safety-and-efficacy-of-benzbromarone-in-gout-in-aotearoa-new-zealand/