Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Background:
Hydroxychloroquine (HCQ) is widely used in treatment of autoimmune rhuematological diseases. In particular, systemic lupus erythermatosus where it proved to prevent disease flare, reduce organ damage and decrease mortality. Although the drug has a favourable safety profile however, it has been associated with increased risk of severe retinal toxicity. Recent data has demonstrated that hydroxychloroquine retinopathy might be more common than previously recognized with an overall prevalence of 7.5%.
Aim:
To reassess and redetermine the overall prevelance of hydroxychloroquine induced retinopathy.
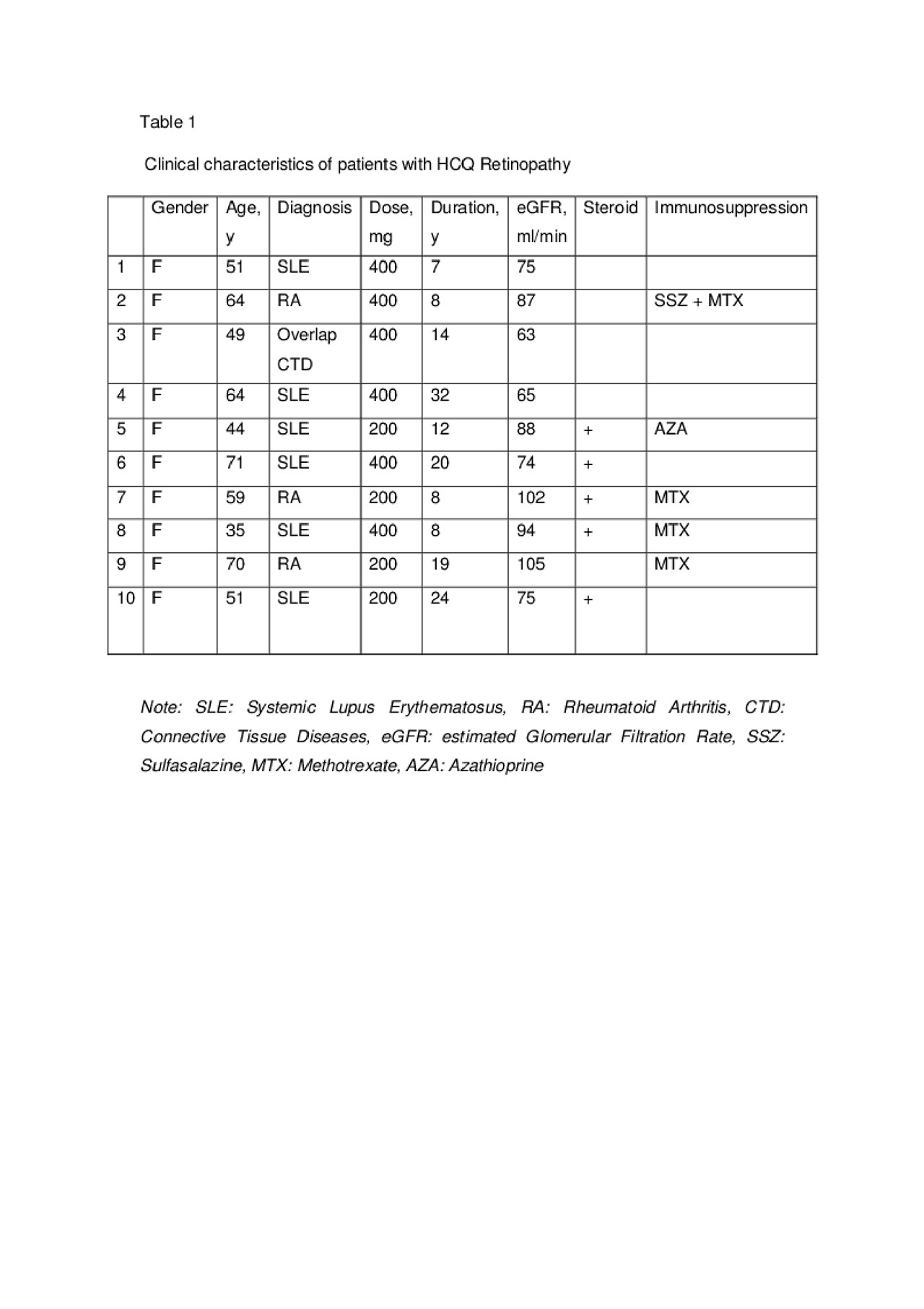
Methods: We retrospectively reviewed the records of 729 patients who were referred to hydroxychloroquine retinopathy screening clinic in the medical eye unit at St Thomas Hospital, London, UK. The following data were collected: age, gender, body weight, indication to use hydroxychloroquine, dosage, duration of treatment, use of steroids or other immunosuppressive therapy, previous kidney disease, estimated glomerular filtration rate (eGFR) and concurrent use of tamoxifen. The analytic statistics was carried out.
Results: A total of 729 patients (88.6% female and 11.4% male) were seen in HCQ retinopathy screening clinic. All patients were on HCQ with mean duration of 7.6 years. The mean age was 48 ±13.9 years (range 13-89). The main indications for HCQ use were systemic lupus erythermatosus (SLE) 50% and rheumatoid arthritis (RA) 17%. Approximately 34% of the patients were on oral corticosteroids and 46% were on conventional and/or biological disease-modifying antirheumatic drugs (DMARDs). Eight patients were on Tamoxifen. The mean HCQ dose was 260 mg (3.9 mg/kg) and the mean eGFR was 83 ml/min. History of renal diseases (lupus nephritis or chronic kidney disease) were reported in 19%, half of them with eGFR less than 60 ml/min. The average daily consumption of HCQ in patients with eGFR less than 30 ml/min was 1.9 mg/kg. Ten patients found to have definitive HCQ retinopathy with overall prevalence 1.4%. The prevalence increased to 2.8% and 5.4% in patients using HCQ for more than 5 years and 20 years or more, respectively. All were female with mean age of 55.8 years (range 35-76). The average daily dosage for patients with retinopathy was 5.17 mg/kg which was higher than patients with normal retinal exam 3.88 mg/kg (P value = 0.1). The mean duration for HCQ use in patients with toxicity was 15.8 years (range 7-32). Renal impairment or tamoxifen use were not observed in our cohort of patients with retinopathy. We found retinopathy is associated with prolonged duration of HCQ use (P< 0.05). The mean duration of HCQ use was 15.2 years in patients with retinopathy compared to 7.5 years for patients with normal retinal exam. In our study, there were no significant correlation between daily HCQ dosage and retinopathy (P=0.903). This might be attributed to the relatively lower daily HCQ dose used in our patient, especially in patients with low eGFR.
Conclusion: In our study, the overall prevalence of HCQ retinopathy was 1.4%, increased to 5.4% in patients using HCQ for 20 years or more. The risk of retinopathy was linked to the prolonged use of HCQ. Lower dosage (2 mg/kg) was found to be safe in patients with low eGFR.
To cite this abstract in AMA style:
Alrashid A, Stone M, Davies N, D'Cruz D. The Risk of Toxic Retinopathy Among Patients on Hydroxychloroquine [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-risk-of-toxic-retinopathy-among-patients-on-hydroxychloroquine/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-risk-of-toxic-retinopathy-among-patients-on-hydroxychloroquine/