Session Information
Date: Sunday, November 13, 2016
Title: Osteoporosis and Metabolic Bone Disease – Clinical Aspects and Pathogenesis - Poster
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Osteoporosis is a common, progressive condition leading to increased bone fragility and susceptibility to fracture. Although osteoporosis therapy decreases fracture risk, fractures while on any current treatment can occur and do not necessarily represent treatment failure. It is therefore of interest to assess whether patients who fracture on denosumab (FREEDOM and FREEDOM Extension) experience a lower risk of subsequent fracture continuing on therapy than those on placebo who have fractured.
Methods: During FREEDOM, postmenopausal women with osteoporosis were randomized to placebo or denosumab for 3 years. During the 7-year Extension, all participants were allocated to receive denosumab. In this analysis, we report subsequent osteoporotic fractures (new vertebral or nonvertebral) in subjects who received ≥2 doses of denosumab during FREEDOM or the Extension, had an osteoporotic fracture while on treatment, and continued treatment post-fracture, compared with subsequent fractures in FREEDOM placebo subjects. These subsequent fractures were analyzed as recurrent events using the stratified Cox model with the robust variance estimation adjusting for prior fracture.
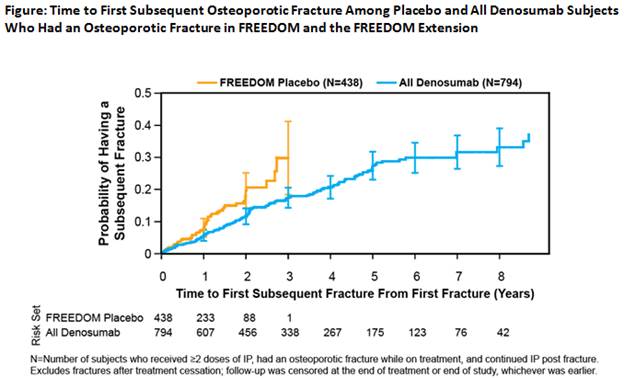
Results: During FREEDOM, 438 placebo and 272 denosumab subjects had an osteoporotic fracture (mean age at first on-study fracture: 74.1 and 74.5 years, respectively). Of these, there were 54 (12.3%) and 24 (8.8%) subjects who had ≥1 subsequent fractures in the placebo and denosumab groups, respectively. Adjusted subject incidence per 100 patient-years was lower for denosumab (6.7) vs placebo (10.1). Combining all subjects on denosumab from FREEDOM and the Extension for up to 10 years, 794 (13.7%) subjects had an osteoporotic fracture while on denosumab (mean age at first on-study fracture: 76.5 years). Of these, one or more subsequent fractures occurred in 144 (18.1%) subjects, with an adjusted subject incidence of 5.8 per 100 patient-years, similar to FREEDOM denosumab (6.7 per 100 patient-years). Among subjects with ≥1 subsequent fracture, 90% had only 1, and spine fracture was most frequent. The risk of having subsequent on-study osteoporotic fractures was lower in all denosumab subjects compared with placebo subjects (HR 0.60 [95% CI: 0.43–0.81]; p=0.0012; Figure).
Conclusion: The risk of a second fracture with continued denosumab treatment remains lower than placebo, thus suggesting that a fracture sustained while on denosumab is not necessarily indicative of a treatment failure, and continuation of treatment should be considered.
To cite this abstract in AMA style:
Kendler D, Chines A, Brandi M, Papapoulos S, Lewiecki E, Reginster JY, Roux C, Munoz Torres M, Wang A, Bone H. The Risk of Subsequent Osteoporotic Fractures Is Decreased in Patients Experiencing Fracture While on Denosumab [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/the-risk-of-subsequent-osteoporotic-fractures-is-decreased-in-patients-experiencing-fracture-while-on-denosumab/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-risk-of-subsequent-osteoporotic-fractures-is-decreased-in-patients-experiencing-fracture-while-on-denosumab/