Session Information
Date: Sunday, November 8, 2015
Title: Spondylarthropathies and Psoriatic Arthritis - Clinical Aspects and Treatment: Treatment of AS
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Conflicting results have been
demonstrated in axial spondyloarthritis (axSpA) with regard to whether
effectiveness of a second (2°) TNFi depends on the reason of discontinuation of
the first (1°) TNFi.
Methods: Patients with a clinical diagnosis of axSpA initiating a 2°
TNFi in the Swiss Clinical Quality Management (SCQM) cohort were included. 2°
TNFi drug maintenance and the proportion of patients achieving a moderately
active or inactive disease state according to defined Ankylosing Spondylitis
Disease Activity Score (ASDAS) cut-offs at 1 year (±6mo) were compared by the
reason of discontinuation of the 1° TNFi (primary or secondary lack of response
(PLR or SLR, defined as discontinuation of the 1° TNFi due to insufficient
effectiveness before or after 6 months, respectively), adverse events (AE) or
other reasons). LUNDEX values were used to indicate the proportion of patients
adhering to treatment and achieving a response criterion.
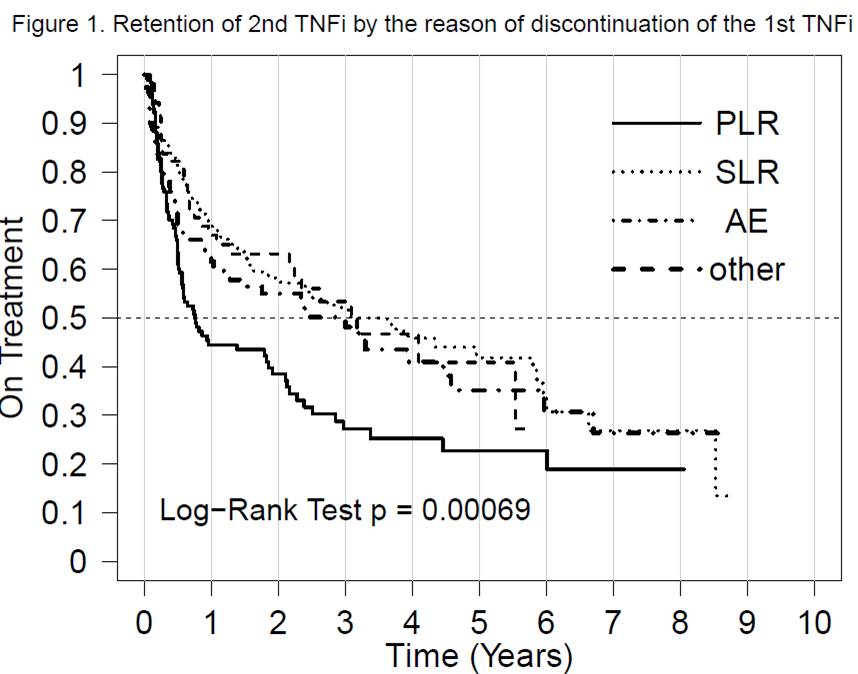
Results: A 2° TNFi was started in 591 patients after inclusion into
SCQM. Drug retention of 2° TNFi stratified by the reason of discontinuation of
the 1° TNFi was significantly reduced after PLR in comparison to all other
reasons of discontinuation, log-rank p<0.001 (Figure 1). Median (IQR) drug
retention after PLR and SLR were 0.94 (0.58; 2.13) and 3.92 (2.46; 3.20) years,
respectively. The proportion of patients achieving an ASDAS <2.1 and
<1.3, respectively, in patients still on treatment (n=384) with complete
follow-up visits at 1 year (n=176) are shown in Table 1. Response rates were
slightly lower in patients having previously experienced PLR and AE in
comparison to SLR and highest in patients having switched due to “other”
reasons (e.g. remission, personal preferences). These differences were partially
more pronounced after LUNDEX adjustment. An ASDAS<1.3 was reached by 2-12%
of patients following a previous PLR, SLR or AE.
Conclusion: Previous PLR is associated with a strongly reduced retention
of the 2° TNFi. Only a minority of patients on a 2° TNFi having previously
experienced PLR, SLR or AE achieve an inactive disease state at 1 year. These
findings might help guiding treatment choices after discontinuation of a 1°
TNFi, as new treatment options with other modes of action will be available.
Table 1. Proportion of patients on a second TNFi
achieving a moderately active or inactive
disease state after 1 year (with and
without LUNDEX adjustement of treatment
responses for drop-outs). #Fisher’s
test; µNon-parametric permutation test.
|
|
All N=176 |
PLR N = 35 |
SLR N= 87 |
AE N=36 |
Other N=18 |
P Value |
|
ASDAS<2.1 |
40% |
34% |
43% |
31% |
61% |
0.16# |
|
ASDAS<2.1 LUNDEX |
28% |
19% |
31% |
21% |
44% |
0.11µ |
|
ASDAS<1.3 |
14% |
13% |
16% |
3% |
28% |
0.052# |
|
ASDAS<1.3 LUNDEX |
10% |
8% |
12% |
2% |
20% |
0.055µ |
To cite this abstract in AMA style:
Ciurea A, Exer P, Weber U, Tamborrini G, Steininger B, Kissling RO, Bernhard J, Scherer A. The Reason of Discontinuation of a First TNF Inhibitor Affects Drug Retention of a Second Anti-TNF Agent in Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/the-reason-of-discontinuation-of-a-first-tnf-inhibitor-affects-drug-retention-of-a-second-anti-tnf-agent-in-axial-spondyloarthritis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-reason-of-discontinuation-of-a-first-tnf-inhibitor-affects-drug-retention-of-a-second-anti-tnf-agent-in-axial-spondyloarthritis/