Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Improved understanding of the complex genetic architecture of PsA along with the reduction in the cost of genetic testing provide an opportunity to assess the application of genetic testing for PsA diagnosis in clinical setting. The study aimed to assess the performance of the multi-SNP genetic test in predicting a clinical diagnosis of PsA by a rheumatologist among psoriasis patients with musculoskeletal symptoms.
Methods: 328 patients with psoriasis and musculoskeletal symptoms that were referred to a rapid access clinic for suspected PsA were enrolled. Patients with a prior diagnosis of PsA were excluded. A rheumatologist evaluated all patients and classified them as “PsA” or “not PsA”. All patients who were classified as not PsA at baseline were reassessed 1 year later to determine whether they have developed PsA. We tested 2 outcomes: 1) diagnosis of PsA at baseline; and 2) diagnosis of PsA at baseline or at 1 year. A custom multi-SNP genetic assay was genotyped on a MassARRAY system (Agena Biosciences). The custom PsA weighted genetic panel included 42 variants in or near 20 genes based on genome-wide significance in PsA studies. We tested the ability of each genetic maker individually to predict PsA using logistic regression models adjusted for age and sex. Machine-learning methods including logistic regression, naïve bayes and random forest were used to identify the optimal prediction model. Age and sex were included in the prediction models.
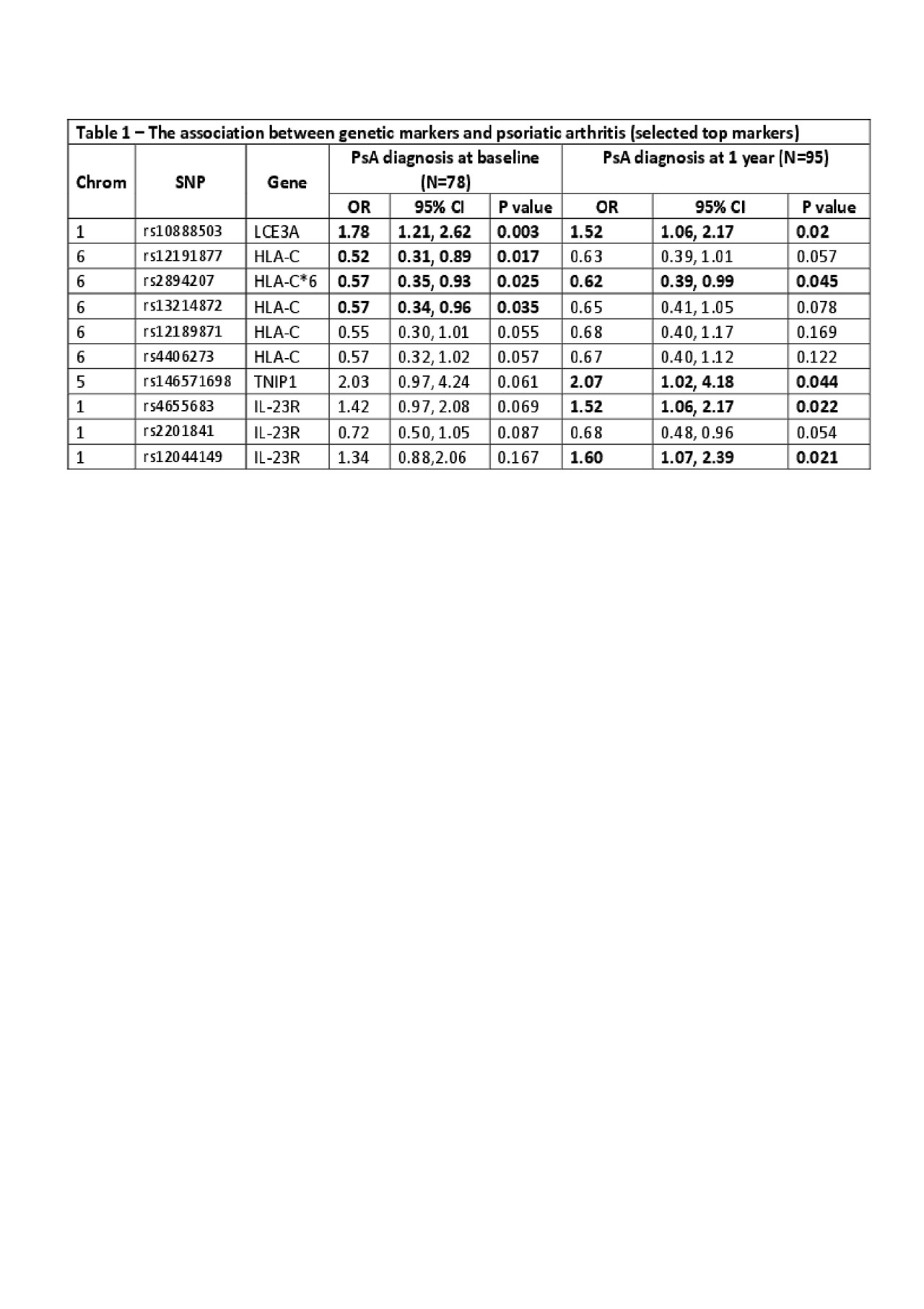
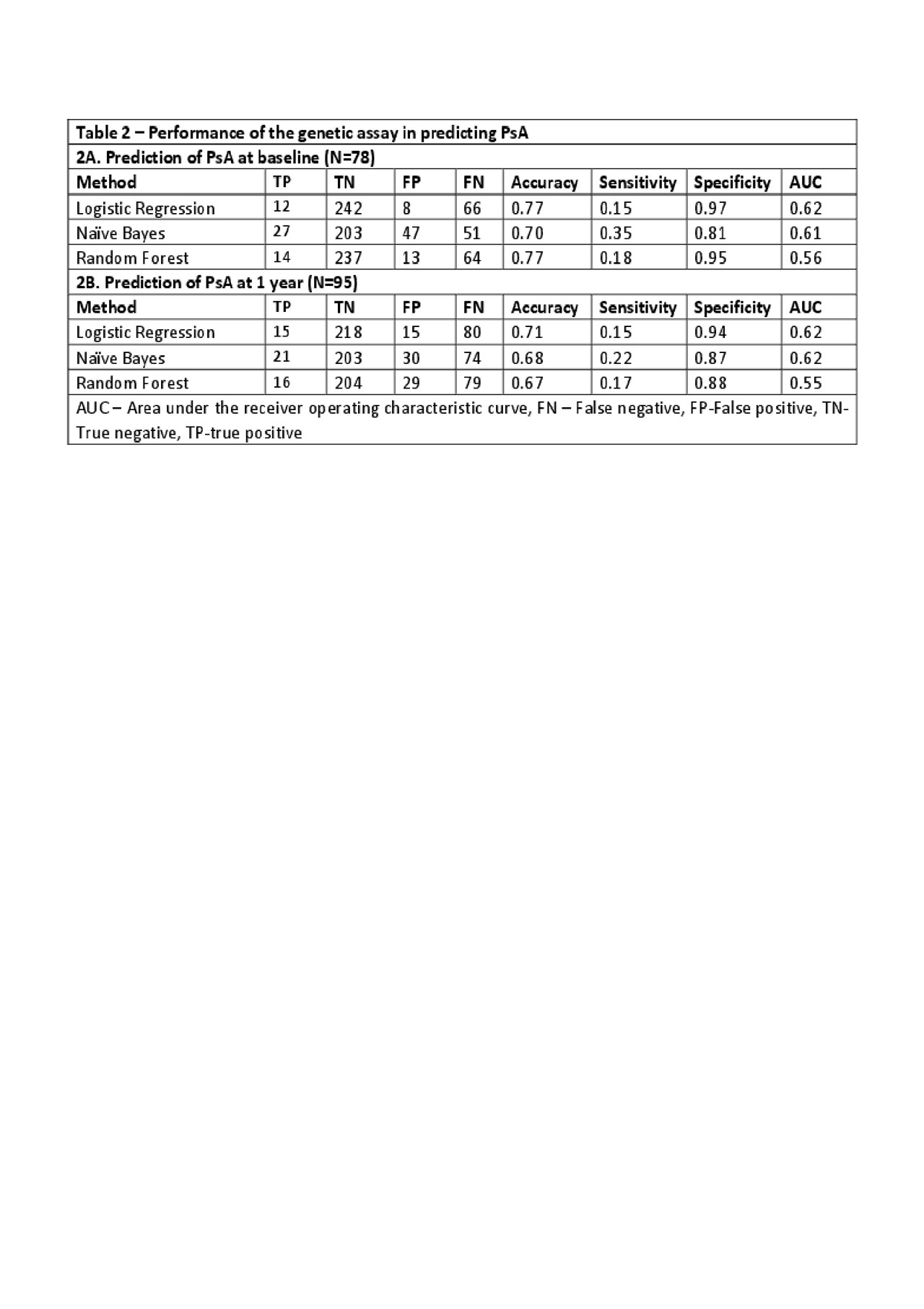
Results: 78 patients were classified as PsA (PsA-baseline) and the remaining 250 patients as not PsA. After 1 year, 17 additional patients developed PsA resulting in 95 patients with PsA at 1 year (PsA-1 year). The association between the tested SNPs and PsA is shown in Table 1. Five SNPs located at LCE3A (rs10888503), TNIP1 (rs146571698) and IL-23R (rs4655683, rs2201841 and rs12044149) were associated with PsA-baseline or PsA-1 year (all p< 0.05). Of the three machine-learning methods used, logistic regression was found to have the best prediction properties (highest AUC). Overall, the performance of prediction models to classify patients as PsA was modest (see Table 2). The AUC, sensitivity and specificity of the models to predict PsA at baseline were: 0.62, 0.15, 0.97, respectively and at 1 year: 0.62, 0.15, 0.94.
Conclusion: Despite the association of several genetic markers with PsA, genetic testing has only a marginal effect on predicting a diagnosis of PsA among patients with psoriasis and musculoskeletal symptoms.
To cite this abstract in AMA style:
Eder L, Li Q, Jerome D, Farrer C, Rahman P, Burry T. The Performance of a Multi-marker Genetic Test to Identify Patients with Psoriatic Arthritis Among Psoriasis Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-performance-of-a-multi-marker-genetic-test-to-identify-patients-with-psoriatic-arthritis-among-psoriasis-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-performance-of-a-multi-marker-genetic-test-to-identify-patients-with-psoriatic-arthritis-among-psoriasis-patients/