Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Tumor necrosis factor (TNF) is a key inflammatory cytokine in the pathogenesis of psoriatic arthritis (PsA), RA, ankylosing spondylitis (AS), and diabetes mellitus (DM). TNF inhibitors (TNFi) have been shown to be associated with a decreased incidence of DM, but it is unknown whether treatment of PsA, RA, or AS with TNFi has off-target therapy benefits for patients with DM. The objective of this study is to determine whether initiation of a TNFi, compared to initiation of MTX or metformin, results in a decrease in Hemoglobin A1c (HbA1c) in patients PsA, RA, or AS with DM and elevated HbA1c.
Methods: A retrospective cohort study was conducted in OptumInsight from 2000-2014, a de-identified administrative claims database that includes laboratory values for approximately 10% of patients. We identified patients with PsA, RA, or AS, and DM (defined by ICD-9-CM codes), with an HbA1c ≥ 7 and examined change in HbA1c among new initiators of a TNFi (etanercept, adalimumab, certolizumab, golimumab, or infliximab), MTX, or metformin (positive control). A baseline period of 12 months prior to the index date was required to capture potential confounders. All patients were required to have one HbA1c in the six months prior to and one HbA1c in the 6 months after drug initiation. We compared median HbA1c change in each treatment group using Wilcoxon Rank Sum (unadjusted). Linear regression models were used to compare change in HbA1c between treatment using MTX as the reference with adjustment for age, sex, baseline A1c, DM medications, and comorbidities in the baseline period, with clustering to account for multiple new drug initiations per patient.
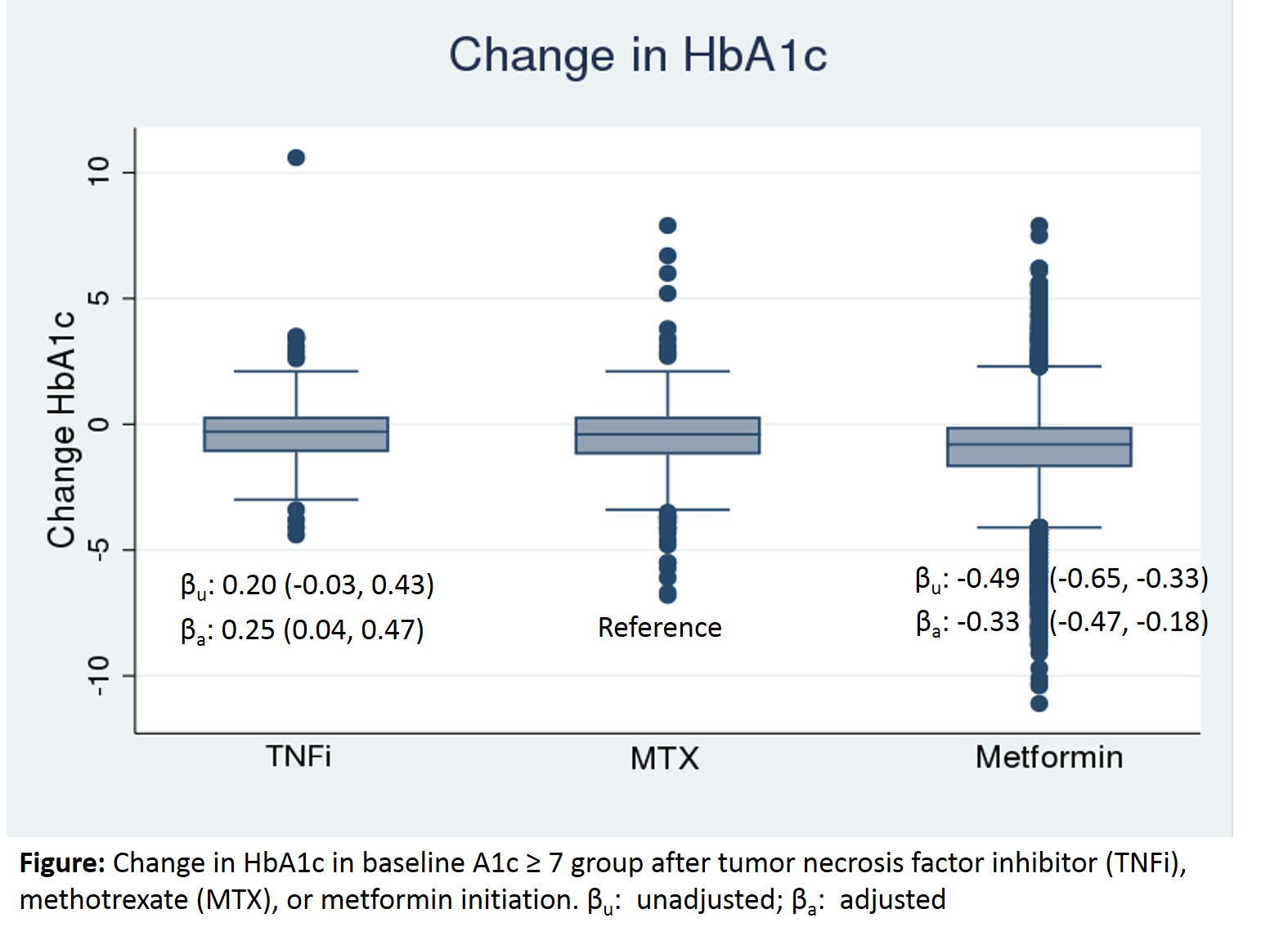
Results: Among 13,135 drug initiations in 12,689 patients with PsA, RA, or AS, diabetes and available HbA1c values, HbA1c was ≥ 7 before 255 (35%) of TNFi initiations, 411 (37%) of MTX initiations, and 5894 (52%) of metformin initiations. The average time between baseline and follow-up HbA1c values was 227 days. Median HbA1c change was -0.30 (IQR -1.10, 0.30) after TNFi initiation, -0.40 (IQR -1.20, 0.30) after MTX initiation, and -0.80 (IQR -1.70, -0.10) after metformin initiation. In adjusted analyses, TNFi initiators had a significantly smaller decrease in HbA1c compared to MTX initiators, β 0.25 (95%CI: 0.04, 0.47), while Metformin initiators had a significantly greater change in HbA1c than MTX patients, β -0.33 (95%CI: -0.47, -0.18).
Conclusion: TNFi and MTX initiation lead to a decline in HbA1c by approximately half as much as metformin.
To cite this abstract in AMA style:
Mantravadi S, George MD, Ogdie A. The Impact of Tumor Necrosis Factor Inhibitors on Diabetes Mellitus Among Patients with Inflammatory Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-tumor-necrosis-factor-inhibitors-on-diabetes-mellitus-among-patients-with-inflammatory-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-tumor-necrosis-factor-inhibitors-on-diabetes-mellitus-among-patients-with-inflammatory-arthritis/