Session Information
Date: Tuesday, November 14, 2023
Title: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment: SpA Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Smoking has been associated with higher disease activity, poorer treatment response, and drug retention rate among psoriatic arthritis (PsA) patients treated with Tumor Necrosis Factor inhibitors. However, few studies have investigated the impact of smoking on secukinumab (an IL17A inhibitor) treatment outcomes. In this study, we therefore aimed to investigate the association between smoking status and 12-month secukinumab retention rate in patients with PsA treated in routine care.
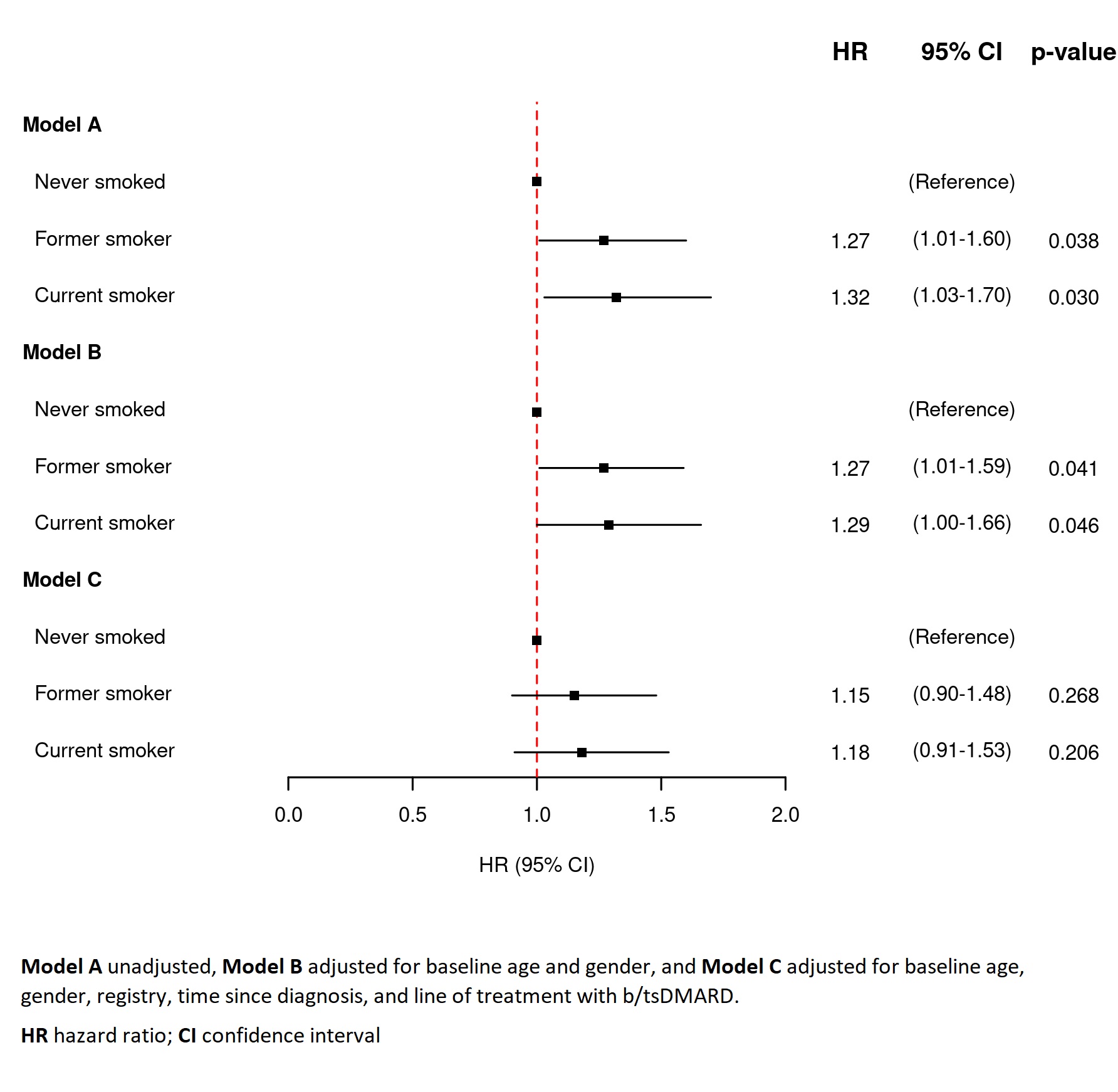
Methods: Patients with PsA, initiating secukinumab between January 1st, 2015 and December 31st, 2020, with available smoking data were identified in 10 European registries participating in the European Spondyloarthritis Research Collaboration Network (EuroSpA RCN). Patients were stratified according to their smoking status (never/former/current) at treatment start (baseline). Kaplan-Meier estimation with log-rank tests and Cox regression with hazard ratios (HR) were performed to assess and compare 12-month secukinumab retention rates. Three regression models were assessed: Model A) unadjusted analyses, Model B) adjusted for baseline age and gender and Model C) further adjusted for registry, time since diagnosis, and line of treatment with biologic/targeted disease-modifying antirheumatic drugs(b/tsDMARD). Missing baseline covariates were imputed using multiple imputation with chained equations.
Results: Of 2,325 patients starting secukinumab treatment, 1,684 had available data on smoking status and were included. At baseline, never smokers compared to former and current smokers were older and scored higher in Disease Activity index for PSoriatic Arthritis in 28 joints (DAPSA28), C-reactive protein (CRP), and Physician Global Assessment, table 1. The 12-month retention rates were 77%, 72% and 71% among never, former, and current smokers, respectively, figure 1. For both former and current smokers, the hazard rates of withdrawal were significantly higher compared to never smokers in unadjusted, and in age and gender-adjusted analyses, figure 2. A similar trend was found in the fully adjusted Model C (HR 1.15 (95% confidence interval 0.90-1.48) and HR 1.18 (0.91-1.53), respectively), figure 2.
Conclusion: In PsA, current and former smokers, who initiate secukinumab treatment appear at increased risk of withdrawing therapy within 12 months as compared to never smokers. Studies of effectiveness should include smoking status, and further studies are needed to investigate if smoking cessation improves treatment outcomes.
To cite this abstract in AMA style:
Faizy Ahmadzay Z, Georgiadis S, Pons M, Hetland M, Glintborg B, Heberg J, Nysom Christiansen S, Horskjær Rasmussen S, Loft A, Castrejon I, Otero-Valera L, Zavada J, Pavelka K, Relas H, Kuusalo L, Moeller B, Nissen M, Rotar Z, Perdan Pirkmajer K, Santos Oliveira D, Cruz-Machado A, Michelsen B, Kristianslund E, Gudbjornsson B, Grondal G, Laas K, Hellamand P, Sari I, Di Giuseppe D, Østergaard M, Ørnbjerg L. The Impact of Smoking Status on One Year Secukinumab Retention Rate in 1,684 Patients with PsA: Real-World Results from the EuroSpA Collaboration [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-smoking-status-on-one-year-secukinumab-retention-rate-in-1684-patients-with-psa-real-world-results-from-the-eurospa-collaboration/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-smoking-status-on-one-year-secukinumab-retention-rate-in-1684-patients-with-psa-real-world-results-from-the-eurospa-collaboration/