Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Depression, a common comorbidity in patients with psoriasis (PsO) and PsA, has been shown to be independently associated with Psoriatic Arthritis Impact of Disease (PsAID-12) scores. We investigated how much active PsO and a positive 2-item Patient Health Questionnaire (PHQ-2) depression screen impacted PsAID-12 scores in patients with PsA in the Understanding Psoriatic Disease Leveraging Insights for Treatment (UPLIFT) survey.
Methods: UPLIFT was a multinational Web-based survey conducted in 2020 in adults who reported ever being diagnosed by a healthcare provider (HCP) with PsA and/or PsO. For patients with PsA, we report PsAID-12 total and domain scores and Patient Acceptable Symptom State (PASS) achievement (PsAID-12 ≤4) according to the presence of active PsO and depression status. A PHQ-2 score ≥3 indicates a positive screen for depression.
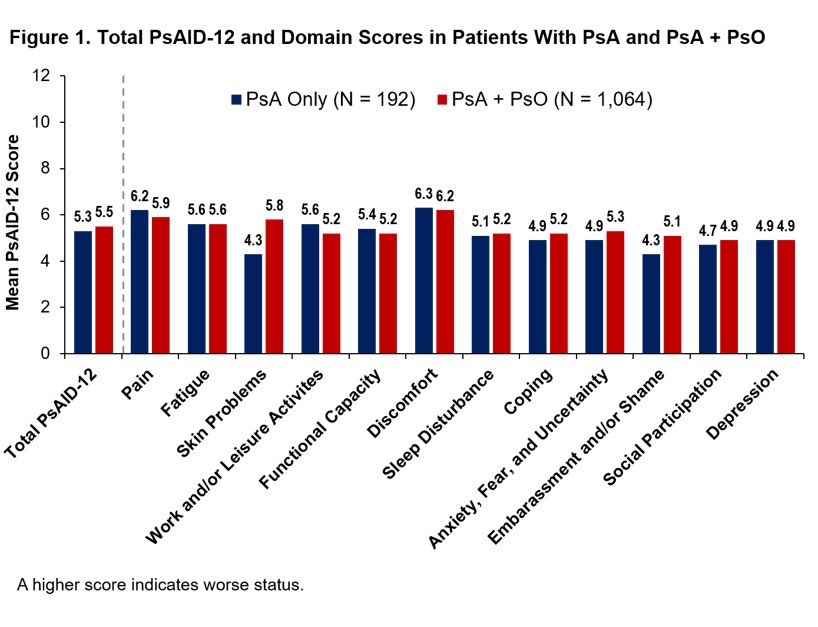
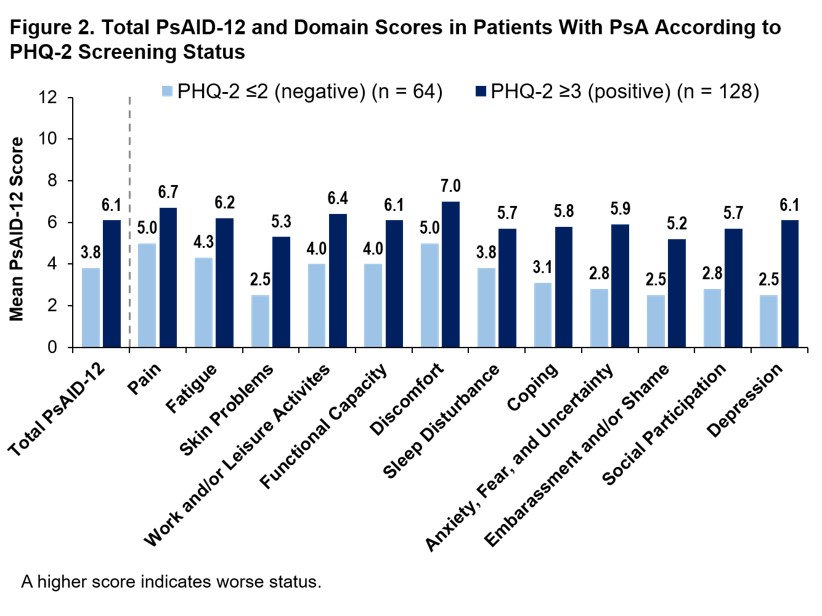
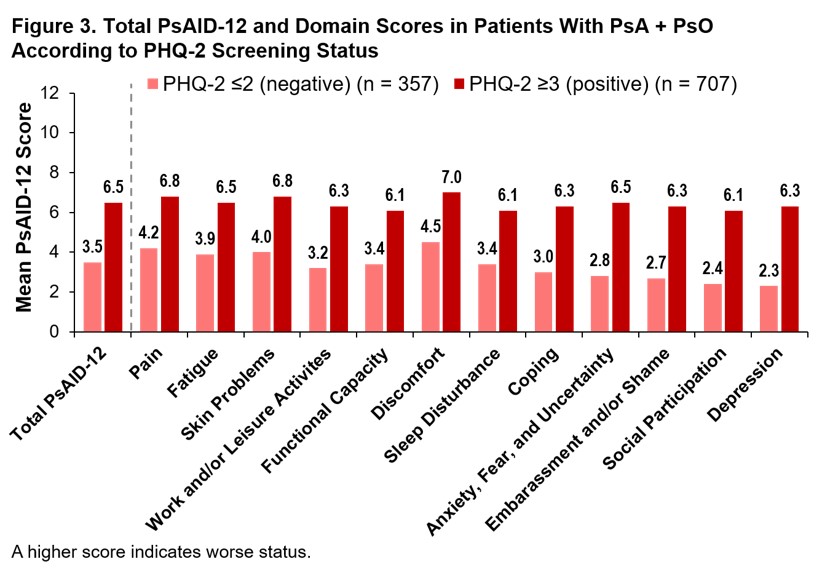
Results: Of the 3,806 patient survey respondents, 1,256 reported HCP-diagnosed PsA, of which 1,064 had HCP-diagnosed PsA + active PsO and 192 had PsA only. In patients with PsA + PsO, enthesitis (44.5%), dactylitis (47.0%), and polyarthritis (59.8%) were more prevalent than in patients with PsA without active PsO (37.5%, 37.5%, and 36.5%, respectively). More patients with PsA + PsO vs PsA only had a history of patient-reported HCP-diagnosed comorbidities, including depression (42.7% vs 26.6%), cancer (30.7% vs 21.4%), heart disease (23.1% vs 13.5%), hypertension (44.0% vs 33.3%), liver disease (21.0% vs 12.5%), and inflammatory bowel disease (25.5% vs 14.1%). Despite differences in comorbidity burden, mean total PsAID-12 scores (Figure 1) and rates of PASS achievement (25.0% vs 26.9%) were similar in patients with PsA only and PsA + PsO. Mean individual PsAID-12 domain scores were generally similar between groups; however, impact of “skin problems” and “embarrassment and/or shame” was more pronounced for the PsA + PsO group (Figure 1). A total of 67% of PsA only and 66% PsA + PsO patients had a PHQ-2 ≥3. Patients with PHQ-2 ≥3 had higher PsAID-12 scores, indicating worse health status, regardless of whether they had active PsO. Fewer patients with PHQ-2 ≥3 achieved PASS (PsA: 10.9%, PsA + PsO: 10.7%) compared with those who screened negative for depression (PsA: 53.1%, PsA + PsO 58.8%). Similarly, higher PsAID-12 domain scores were reported in patients with PsA with PHQ-2 ≥3 vs those with PHQ-2 ≤2 (Figure 2) and to a greater extent in those with PsA + PsO (Figure 3). The greatest differences between groups were observed in domains of “work and/or leisure activities,” “coping,” “anxiety, fear, and uncertainty,” “embarrassment and/or shame,” and “social participation.”
Conclusion: For patients with PsA in UPLIFT, active PsO was associated with generally more active disease and a higher prevalence of comorbidities, including depression. Having a positive PHQ-2 screen for depression was associated with overall disease impact as measured by PsAID.
To cite this abstract in AMA style:
Ogdie-Beatty A, Merola J, Richette P, Richter S, Jardon S, Tang L, Tillett W. The Impact of Skin Involvement and Depression on Patient Acceptable Symptom State in Patients with Psoriatic Arthritis and Psoriasis: Results from a Multinational Survey [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-skin-involvement-and-depression-on-patient-acceptable-symptom-state-in-patients-with-psoriatic-arthritis-and-psoriasis-results-from-a-multinational-survey/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-skin-involvement-and-depression-on-patient-acceptable-symptom-state-in-patients-with-psoriatic-arthritis-and-psoriasis-results-from-a-multinational-survey/