Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriatic arthritis (PsA) is a heterogeneous, chronic, immune mediated disease associated with psoriasis (PsO), with varying skin severity which can range from mild to severe involvement. It is not well understood how skin severity can impact treatment outcomes.
Here we evaluate the impact of PsO severity on clinical, functional, and structural outcomes among PsA pts treated with adalimumab (ADA) or placebo (PBO).
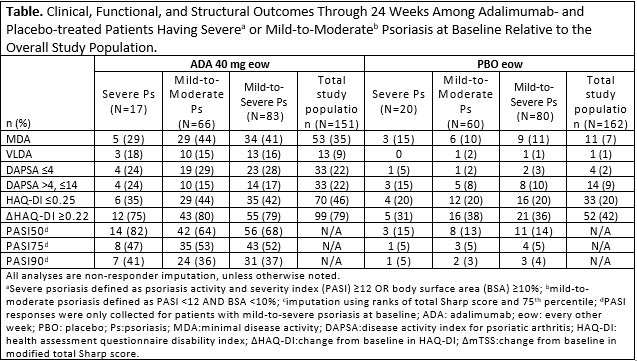
Methods: Pts with baseline PASI and BSA available from ADEPT, a phase 3, randomized, double-blind, PBO-controlled trial of ADA 40 mg every other week, were included in this post hoc analysis.2 Pts were sub-grouped based on baseline PsO severity (severe PsO: PASI ≥12 OR BSA ≥10%; mild-to-moderate PsO: PASI < 12 AND BSA < 10%), relative to overall study population. Clinical outcomes at wk 24 were assessed by attainment of minimal disease activity (MDA), remission and low disease activity (LDA) by disease activity index for psoriatic arthritis (DAPSA), and PASI50/75/90; function and structural progression were assessed by the % of pts experiencing HAQ-DI normative values (≤0.25) and improvement from baseline ≥0.22 (MCID), and the % of pts having change from baseline in mTSS ≤0 and ≤0.5 in the modified total Sharp score (mTSS) respectively. A logistic model with treatment group baseline PASI/BSA and PASI/BSA by treatment interaction was fit for each outcome variable. P-values for interaction term and baseline BSA/PASI were calculated. Non-responder imputation was used for missing clinical and functional endpoints. 75th percentiles were imputed for missing radiographic data. Treatment-emergent adverse events (TEAEs) were monitored throughout.
Results: Of the 163 pts enrolled in the study with baseline PASI and BSA, only 23% (n=37;ADA: 17; PBO: 20) were classified as having severe PsO and had similar characteristics to those with mild-to-moderate PsO, except for numerically higher physician’s global assessment, dactylitis, and enthesitis scores. Following 24 wks of treatment, 41% of ADA-treated pts achieved MDA and 45% achieved DAPSA LDA or better, a finding consistent irrespective of baseline PsO severity (Table). 72% of ADA-treated pts with baseline PsO did not exhibit any structural progression by mTSS (≤0) through 24 wks as compared to 55% receiving PBO. Logistic regression analyses confirmed limited role that PsO severity played across examined outcomes. DAPSA remission was more likely at wk 24 among pts with higher baseline PASI scores, and this effect was less apparent with ADA treatment. TEAEs appeared comparable between ADA and PBO groups; fewer infectious AEs occurred in the ADA severe PsO group vs the mild-to-moderate PsO group.
Conclusion: Prevalence of severe PsO in this population of PsA pts was consistent with previous observations.3 Treatment with ADA was associated with better PsA outcomes as compared to PBO irrespective of the degree of PsO severity at baseline.
1. Singh, et al. Arthritis Rheumatol, 2018;71:2-29.
2. Mease, et al. Arthritis Rheumatol, 2005;52:3279-89.
3. Alinaghi, et.al. J. Am. Acad. Der, 2019;80-1-:251-265.e219.
To cite this abstract in AMA style:
Merola J, Coates L, Lesser E, Valdecantos W, Song Y, Mease P. The Impact of Psoriasis Severity on Outcomes Among Psoriatic Arthritis Patients Receiving Adalimumab [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-impact-of-psoriasis-severity-on-outcomes-among-psoriatic-arthritis-patients-receiving-adalimumab/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-psoriasis-severity-on-outcomes-among-psoriatic-arthritis-patients-receiving-adalimumab/