Session Information
Date: Tuesday, October 28, 2025
Title: (2195–2226) Reproductive Issues in Rheumatic Disorders Posters
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is associated with a higher risk of adverse pregnancy outcomes (APOs) compared to the general population. Although several APO risk factors have been identified in SLE pregnancies, standardized criteria for pregnancy planning remain undefined. The 2024 EULAR guidelines recommend tapering glucocorticoids to a prednisolone-equivalent dose of ≤5 mg/day prior to conception; however, the optimal threshold for pregnancy planning remains unclear. This study aimed to address these gaps.
Methods: This retrospective cohort study included patients with SLE who received care and delivered at three tertiary referral centers in Japan. Logistic regression and receiver operating characteristic (ROC) curve analyses were used to identify preconception factors associated with APOs. Odds ratios (ORs) were calculated based on pregnancy planning status.
Results: We analyzed 130 pregnancies in 123 women. Based on exploratory analysis, planned pregnancy was defined by the absence of teratogenic medications, clinical disease inactivity for ≥6 months before conception, prednisolone dosage ≤5 mg/day, and absence of proteinuria (≤0.5 g/day). According to this definition, 44.6% (58/130) was classified to planned pregnancy. This model was associated with significantly lower APO rates. Compared to unplanned pregnancies, planned pregnancies had reduced overall APOs (OR 0.29, 95% CI 0.13–0.61, p = 0.0012), maternal APOs (OR 0.50, 95% CI 0.22–1.13, p = 0.097), neonatal APO (OR 0.33, 95% CI 0.16–0.68, p = 0.0026), and low birth weight (OR 0.46, 95% CI 0.21–1.01, p = 0.053). Multivariate analysis adjusted for hydroxychloroquine use, aspirin use, and renal involvement confirmed a lower risk of APOs: overall APO (aOR 0.30, 95% CI 0.13–0.70, p = 0.0055), maternal APO (aOR 0.50, 95% CI 0.19–1.27, p = 0.15), and neonatal APO (aOR 0.39, 95% CI 0.17–0.86, p = 0.019). Additionally, analysis using multiple models demonstrated that a prednisolone dosage ≤5 mg/day at conception was not significantly associated with an increased risk of APOs or SLE flares during pregnancy, even when compared with higher dosage thresholds.
Conclusion: Planned pregnancy is significantly associated with improved outcomes in SLE. These findings support the 2024 EULAR recommendation and highlight the importance of structured preconception planning
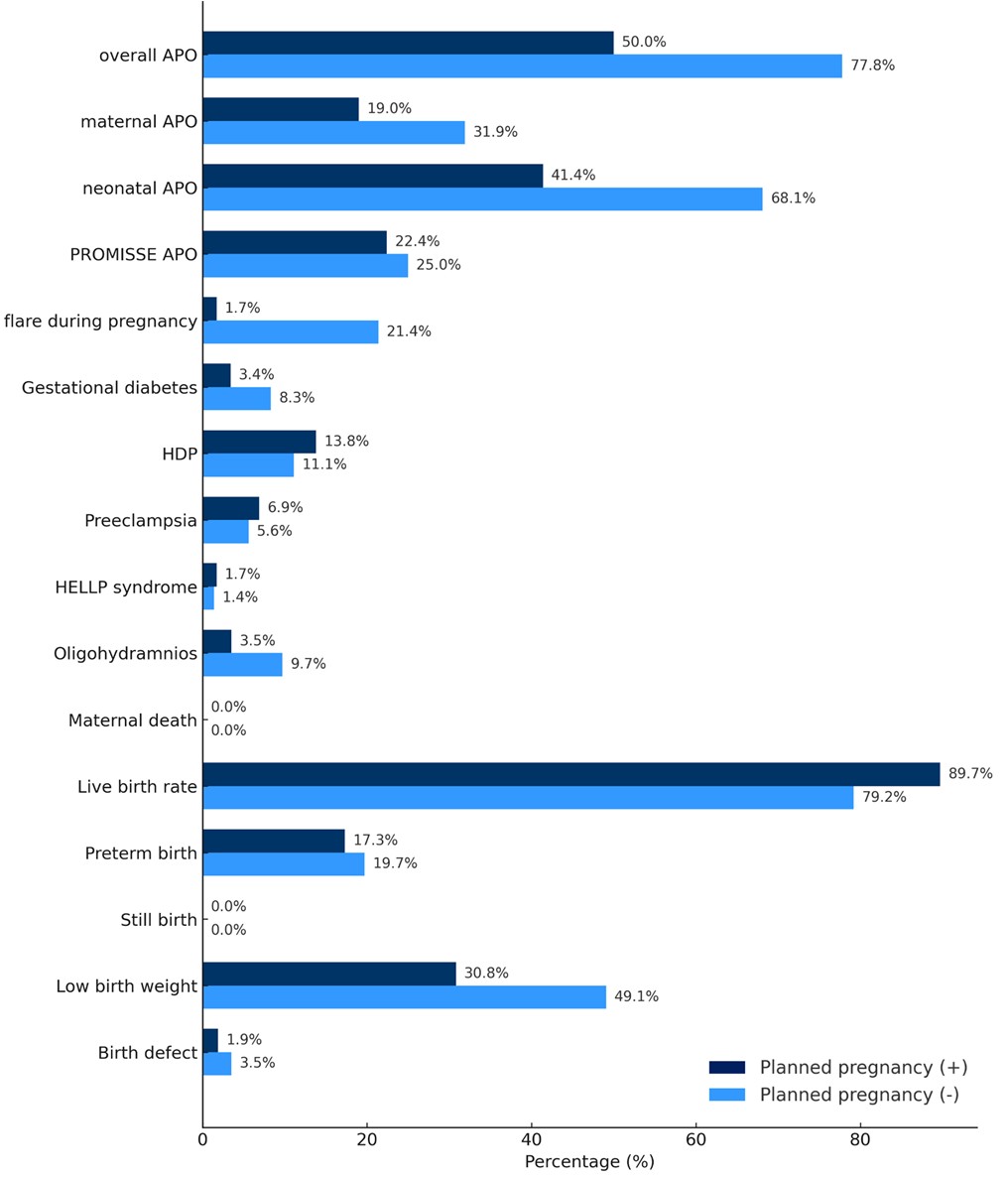 Figure 1. prevalence of each adverse pregnancy outcome according to pregnancy planning
Figure 1. prevalence of each adverse pregnancy outcome according to pregnancy planning
.jpg) Table 1. Baseline characteristics
Table 1. Baseline characteristics
.jpg) Table 2. Adverese pregnancy outcome ratio according to pregnancy planning
Table 2. Adverese pregnancy outcome ratio according to pregnancy planning
To cite this abstract in AMA style:
Nakai T, Honda N, HASHIMOTO Y, Soga E, Fukui S, Kitada A, Nakanishi K, Yokogawa N, Okada M. The Impact of Pregnancy Planning on the Prevalence of Adverse Pregnancy Outcomes in Women with Systemic Lupus Erythematosus: A Retrospective Study from Three Japanese Tertiary Referral Centers [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-pregnancy-planning-on-the-prevalence-of-adverse-pregnancy-outcomes-in-women-with-systemic-lupus-erythematosus-a-retrospective-study-from-three-japanese-tertiary-referral-centers/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-pregnancy-planning-on-the-prevalence-of-adverse-pregnancy-outcomes-in-women-with-systemic-lupus-erythematosus-a-retrospective-study-from-three-japanese-tertiary-referral-centers/
