Session Information
Session Type: ACR/ARHP Combined Abstract Session
Session Time: 9:00AM-11:00AM
Background/Purpose: The AbbVie patient (pt) support program (PSP) is offered to pts prescribed adalimumab for RA and other indications. The purpose of this analysis was to evaluate the incidence of disease flare and the regain of disease control after flare among pts treated with adalimumab in the PASSION study according to PSP participation.
Methods: Pts with moderate to severe RA were enrolled in a 78-wk postmarketing, global, observational study (PASSION) and received adalimumab in routine clinical care. Pts achieving low disease activity (LDA), defined as a 28-joint Disease Activity Score using C-reactive protein (DAS28[CRP]) ≤3.2, were evaluated for the incidence of disease flare and recovery of LDA through wk 78. Disease flare was defined as an increase in DAS28(CRP) ≥0.6 at 2 consecutive visits (current and previous visits) and DAS28(CRP) >3.2 at either visit if pts had achieved DAS28(CRP) LDA or better at or before the previous visit. The proportion of PSP users and PSP non-users with LDA experiencing disease flare and the proportion who re-established LDA following disease flare were summarized by visit. Differences between PSP users and PSP non-users were evaluated using a Chi-square test.
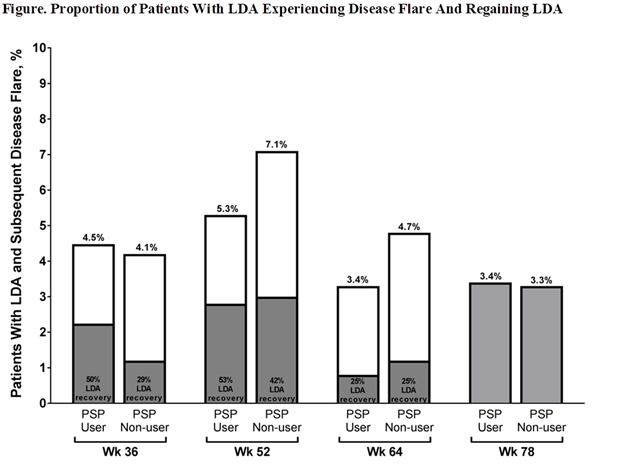
Results: Of the 1025 pts enrolled in PASSION, 695 pts achieved LDA through wk 78 (357/499 [72%] PSP users; 338/526 [64%] PSP non-users); 124 (18%) of whom experienced disease flare at ≥1 time point (59/357 [17%] PSP users; 65/338 [19%] PSP non-users). Of pts who experienced a flare, similar proportions of PSP users and PSP non-users were female (46/59 [78%] PSP users; 55/65 [85%] PSP non-users) and <65 years (48/59 [81%] PSP users; 46/65 [71%] PSP non-users); however, significant differences (P<0.001) were observed for race (white; 44/59 [75%] PSP users; 64/65 [99%] PSP non-users), ethnicity (Hispanic/Latino; 17/59 [29%] PSP users; 3/65 [5%] PSP non-users), and prior biologic DMARD use (4/59 [7%] PSP users; 22/65 [34%] PSP non-users). Flares occurred in similar proportions of PSP users (3.4%–5.3%) and PSP non-users (3.3%–7.1%) with LDA at assessed time points (Figure). Greater portions of PSP users (50%–53%) vs PSP non-users (29%–42%) regained LDA after flare through wk 52; 12 PSP users and 11 PSP non-users experienced disease flare at wk 78 and were unable to be assessed for LDA achievement following flare.
Conclusion: Flares occurred in <20% of pts receiving adalimumab and achieving LDA in the 78-wk PASSION study. Greater proportions of PSP users than PSP non-users regained disease control after flare through study wk 52.
Acknowledgment: AbbVie funded the study, contributed to the design, collection, analysis, and interpretation of the data, and in the writing, review, and approval of the abstract. Medical writing support was provided by Catherine DeBrosse, PhD, and Janet Matsuura, PhD, of Complete Publication Solutions, LLC (North Wales, PA) and was funded by AbbVie.
To cite this abstract in AMA style:
van Den Bosch F, Wassenberg S, Haraoui B, Zueger P, Wu M, Lagunes Galindo I, Ostor A. The Impact of Participation in an Adalimumab (Humira) Patient Support Program on the Onset and Management of Disease Flares [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-participation-in-an-adalimumab-humira-patient-support-program-on-the-onset-and-management-of-disease-flares/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-participation-in-an-adalimumab-humira-patient-support-program-on-the-onset-and-management-of-disease-flares/