Session Information
Date: Saturday, November 6, 2021
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Impact (0225–0240)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Patients (pts) with PsA experience pain, loss of physical function, joint damage, and significant impairments in social and emotional well-being. The Short Form Health Survey 36-item questionnaire (SF-36v2), a generic measure of pt-reported health-related quality of life (HRQOL), includes 36 items and measures 8 domains—physical functioning (PF), role-physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role-emotional (RE), and mental health (MH)—that contribute to both physical component summary (PCS) and mental component summary (MCS) scores. Deucravacitinib is a novel, oral selective inhibitor of tyrosine kinase 2 (TYK2), an intracellular kinase that mediates cytokine signaling pathways implicated in the pathogenesis of PsA. In a Phase 2 trial in pts with active PsA, the primary endpoint, ACR 20 response at Week (Wk) 16, was met and deucravacitinib was well tolerated versus placebo (PBO).1 This analysis further evaluated the effect of deucravacitinib treatment on SF-36 scores.
Methods: This 1-year, randomized, double-blind, placebo-controlled, multicenter Phase 2 trial (NCT03881059) enrolled pts with a PsA diagnosis ≥6 months who fulfilled Classification Criteria for Psoriatic Arthritis at screening and had active joint disease (≥3 tender and ≥3 swollen joints), high-sensitivity CRP ≥3 mg/L, and ≥1 plaque psoriasis lesion (≥2 cm). Pts failed or were intolerant to ≥1 NSAID, conventional synthetic DMARD, and/or 1 TNF inhibitor (TNFi; ≤30%). Pts were randomized 1:1:1 to receive deucravacitinib 6 mg once daily (QD) or 12 mg QD, or PBO. Changes from baseline (BL) in SF-36 PCS and MCS scores at Wk 16 were prespecified key secondary and additional endpoints, respectively. Analyses evaluated the 8 SF-36 domain scores at Wk 16. The proportion of patients who reported improvements ≥2.5 and ≥5-points (the minimum clinically important difference [MCID]) in SF-36 summary and domain scores, respectively, were evaluated.
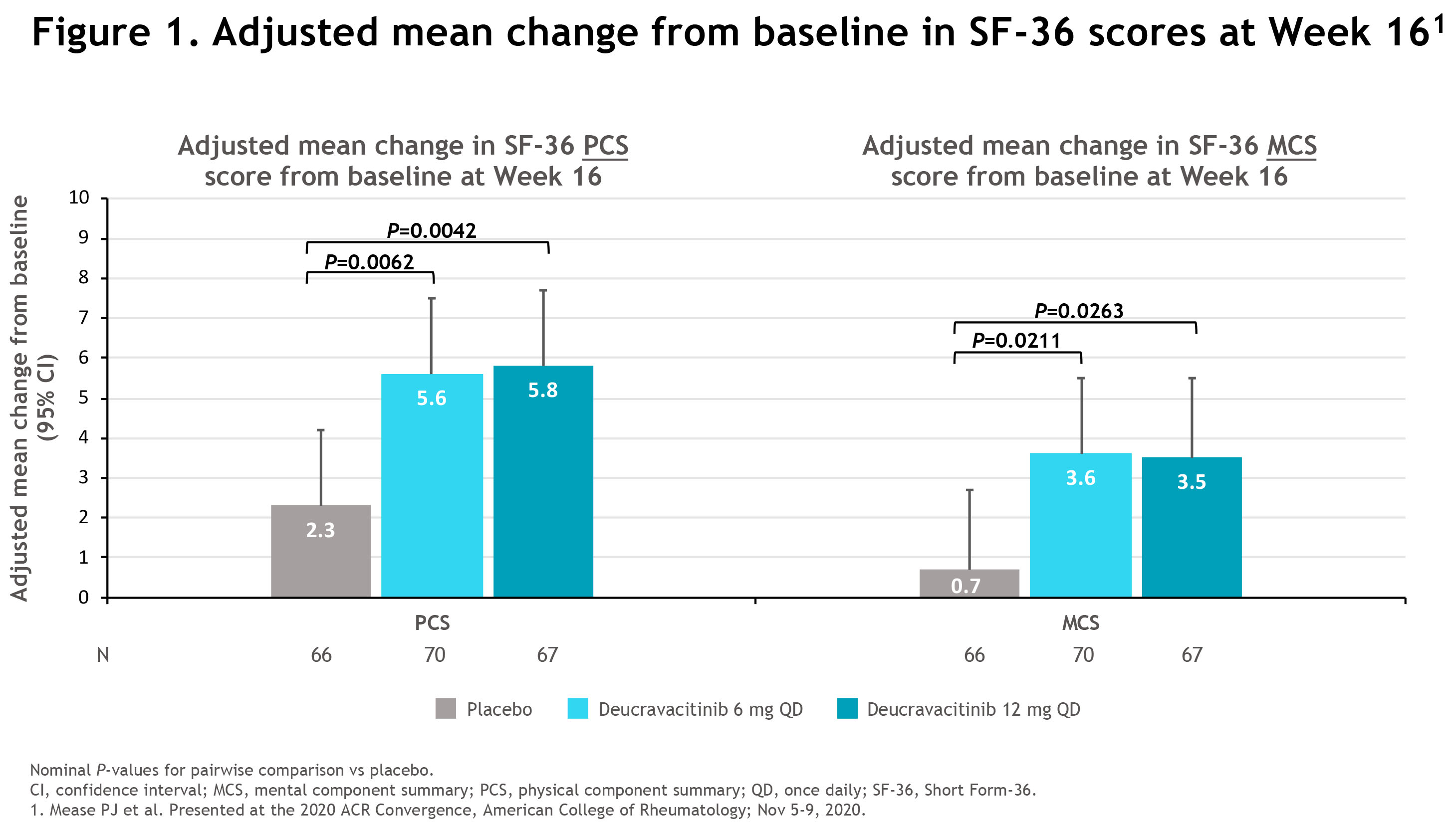
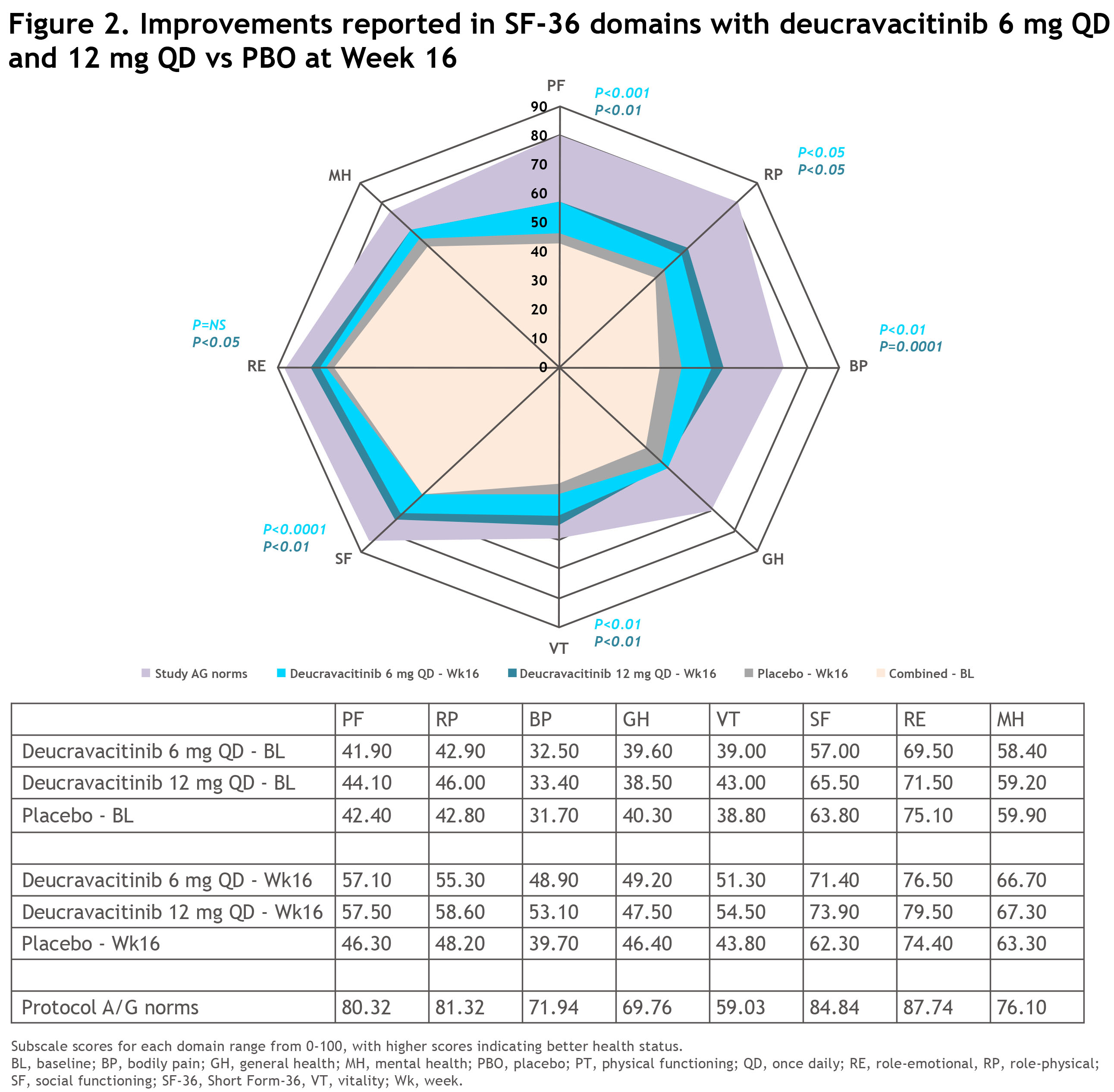
Results: Of 203 pts randomized, 180 (89%) completed 16 wks of treatment (deucravacitinib 6 mg QD, 63/70 [90%]; deucravacitinib 12 mg QD, 59/67 [88%]; PBO, 58/66 [88%]). Demographic and BL disease characteristics were similar across groups. Mean age was 49.8 years and median PsA duration since diagnosis was 4.5 years. BL mean SF-36 PCS and MCS scores were similar among deucravacitinib 6 mg QD, 12 mg QD, and PBO groups (PCS: 34.0, 34,5, and 33.4; MCS: 45.4, 46.9, and 47.5, respectively; Table). At Wk 16, adjusted mean changes from BL in SF-36 PCS and MCS scores were significantly improved (P< 0.05) with deucravacitinib 6 and 12 mg QD treatment vs PBO (Figure 1). Reported improvements in domain scores with both doses exceeded MCID and were significant in 5 of 8 domains with deucravacitinib 6 mg QD (PF, RP, BP, VT, and SF) and 6 of 8 domains with deucravacitinib 12 mg QD (RE in addition; Figure 2).
Conclusion: Pts with PsA receiving deucravacitinib treatment reported clinically meaningful and significant improvements in HRQOL, including fatigue and social functioning in addition to physical functioning and pain, at Wk 16.
Reference: 1. Mease PJ et al. Presented at the 2020 ACR Convergence, American College of Rheumatology; Nov 5-9, 2020.
To cite this abstract in AMA style:
Strand V, Mease P, Deodhar A, Ye J, Nowak M, Choi J, Becker B. The Impact of Deucravacitinib on Health-Related Quality of Life Measured by the Short Form Health Survey 36-Item Questionnaire: Analysis of a Phase 2 Trial in Patients with Active PsA [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-deucravacitinib-on-health-related-quality-of-life-measured-by-the-short-form-health-survey-36-item-questionnaire-analysis-of-a-phase-2-trial-in-patients-with-active-psa/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-deucravacitinib-on-health-related-quality-of-life-measured-by-the-short-form-health-survey-36-item-questionnaire-analysis-of-a-phase-2-trial-in-patients-with-active-psa/