Session Information
Date: Tuesday, November 14, 2023
Title: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment: SpA Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatologists have access to a plethora of advanced biologic and small molecule agents for the treatment of psoriatic arthritis (PsA). Prior to FDA approval, products undergo extensive clinical trial research vital in determining various outcome measures, such as a product’s safety and efficacy. The knowledge gleaned from trial metrics empowers physicians to make informed treatment decisions for their patients. This research sought to understand brand performance, prescribing habits, and the influence of various clinical trial response criteria in treatment decisions.
Methods: An independent market analytics firm collaborated with US rheumatologists (n=101) to conduct analysis of the PsA market. Data were collected via an online survey fielded May 11 through May 23, 2023, including physician demographics, product usage, and attitudinal survey responses.
Results: Tumor necrosis factor (TNF) inhibitors continue to dominate the psoriatic arthritis market, capturing the majority of share in the first and second-line settings. Physicians reach for alternative mechanisms of action more frequently in second and later lines, led by Interleukin-17 (IL-17) inhibitors.
Two-fifths of surveyed rheumatologists indicate head-to-head studies comparing pipeline assets to adalimumab would be the most compelling as opposed to trials comparing any other pharmacologic treatment; preference for adalimumab as a comparator is unsurprising given physicians’ experience, as well as the agent’s long-term safety and efficacy data.
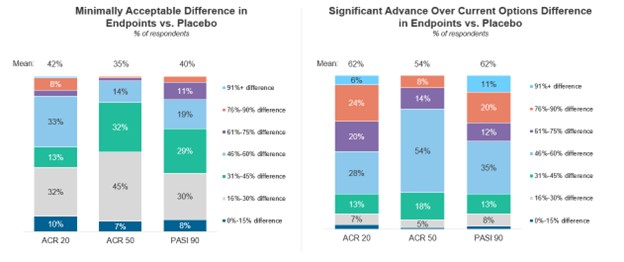
When reviewing placebo-controlled clinical trials, rheumatologists consider 42% ACR20, 35% ACR50, and 40% PASI90 responses as a minimally acceptable improvement. Although physicians do not indicate a strong preference towards one primary clinical trial endpoint, ACR50 responses and minimal disease activity (MDA) are most favorable. ACR70 responses are preferred as secondary endpoints.
With the upcoming launches of secukinumab IV and bimekizumab, physicians express the most interest in gaining access to an IV IL-17 inhibitor. Rheumatologists report having an IV formulation available will fill a number of unmet needs in PsA, most notably access for Medicare patients.
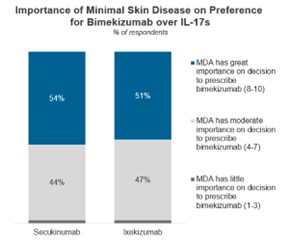
Bimekizumab’s long-term data released from the BE OPTIMAL-3 study has an extremely positive impact on physician perceptions as well as their willingness to prescribe the IL-17A/F inhibitor. Half of those surveyed cite MDA as very influential in determining whether to prescribe bimekizumab over the currently available IL-17 inhibitors for the treatment of PsA.
Conclusion: Physicians express most interest in ACR50 and minimal disease activity as primary endpoints and prefer adalimumab as a comparator in head-to-head studies. The composition of clinical trials, from their primary endpoints to their outcome measurements, influence rheumatologists’ prescribing habits in the PsA market.
To cite this abstract in AMA style:
Yarnall M, Murray K. The Impact of Clinical Trial Metrics in Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/the-impact-of-clinical-trial-metrics-in-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-impact-of-clinical-trial-metrics-in-psoriatic-arthritis/