Session Information
Date: Sunday, October 21, 2018
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The antiphospholipid syndrome (APS) is defined by vascular thrombosis or pregnancy morbidity in the presence of persistently circulating antiphospholipid antibodies (aPL). Those positive for multiple types of aPL have greatest future thrombotic risk. Little is known regarding testing patterns in a population-based sample and whether testing for aPL is done in accordance with the revised Sydney recommendations. We characterized patterns of aPL testing in a large general population sample.
Methods: We performed a retrospective analysis using Truven Health MarketScan® Research Databases, that integrate de-identified patient health data (contributed by large employers, managed care organizations, hospitals, EMR providers, Medicare and Medicaid) including laboratory results on a subset (over 1 million). We used MarketScan lab data from 2010-2015 to identify individuals tested for lupus anticoagulant (LA), anti-cardiolipin (aCL), and anti-beta2-glycoprotein1 (aGP1) antibodies. All subjects were required to be at least 18 years old, having continuous eligibility for medical benefits at least 12 months before and 3 months following the first aPL test.
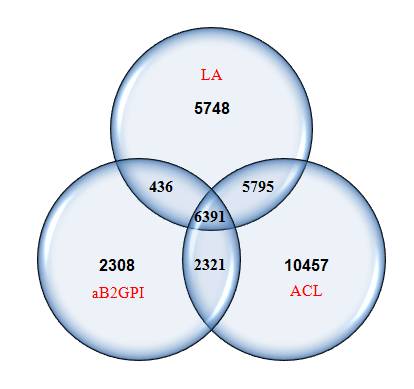
Results: We identified 33,456 individuals who had had at least one aPL test performed. The distribution of testing is shown in Figure 1. In these 33, 456 individuals, only 6,391 (19%) had been tested for all three tests (LA, aCL, aGP1). Of those 33,456 tested at least once, 5786 (17.3%) were positive for at least one test and 255 of these 5,786 (10.6%) had a confirmatory second finding.
18,370 individuals had one or more LA test, among which 1,291 (7%) were positive initially. Among these 1,291 initially positive, only 996 (77%) were known to have been retested ≥12 weeks later.
24,964 individuals had one or more aCL test, among whom 3,753 (15%) were positive initially. Of those 3,753 initially positive, only 1,707 (45%) were re-tested ≥12 weeks.
11,456 individuals had one or more aGP1 test, among whom 1,304 (11%) were positive initially. Of those 1,304 initially positive, only 537 (41%) were re-tested ≥12 weeks.
Conclusion: In this retrospective claims analysis, and applying revised Sydney criteria for APS diagnosis, we determined that confirmatory aPL testing was performed ≥12 weeks in 77%, 45%, and 41% of initially positive LA, aCL, and aGP1, respectively. In addition, in those individuals who had been tested at least once for any aPL, only 6,391 had been tested for all 3 aPL. These findings highlight that aPL testing may often be incompletely performed. Limitations of these analyses include the possibility that testing was done outside the window for which we had data. Further characterization of testing patterns in individuals with known thrombotic events, pregnancy morbidity, systemic lupus, and other high-risk conditions is warranted and may help to inform local diagnostic practices.
To cite this abstract in AMA style:
Egiziano G, Widdifield J, Rahman A, Vinet E, Moura CS, Curtis JR, Bernatsky S. The Frequency of Screening and Prevalence of Antiphospholipid Antibodies in the General Population [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/the-frequency-of-screening-and-prevalence-of-antiphospholipid-antibodies-in-the-general-population/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-frequency-of-screening-and-prevalence-of-antiphospholipid-antibodies-in-the-general-population/