Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
The efficacy and safety of certolizumab pegol (CZP)+methotrexate (MTX) therapy compared to MTX alone, in Japanese MTX-naïve early rheumatoid arthritis (RA) patients (pts) with poor prognostic factors from the C-OPERA trial, have not been previously reported.
Methods
Pts with ≤12 months persistent arthritis fulfilling 2010 ACR/EULAR classification criteria1 were enrolled in this multicenter, double-blind, randomized study (NCT01451203). Eligible pts were MTX-naïve, with at least moderate disease activity (DAS28[ESR]≥3.2), high-positive anti-CCP (>3xULN), and were either rheumatoid factor positive or had bone erosion on radiographs of hands/feet. Pts were randomized 1:1 to CZP+MTX or placebo (PBO)+MTX. Unless precluded by tolerability, MTX dose was rapidly escalated to 16mg by Week (Wk) 8. The primary endpoint was change from baseline (CFB) in van der Heijde modified total Sharp score (mTSS) at Wk52. Secondary endpoints were CFB in mTSS at Wk24, and clinical remission rates by DAS28[ESR], ACR/EULAR (SDAI), and ACR/EULAR (Boolean) at Wk24 and 52. Post-hoc analyses assessed whether higher radiographic progression amongst PBO+MTX treated pts occurred within certain categories of baseline (BL) factors (eg. CRP, HAQ-DI, MMP-3).
Results
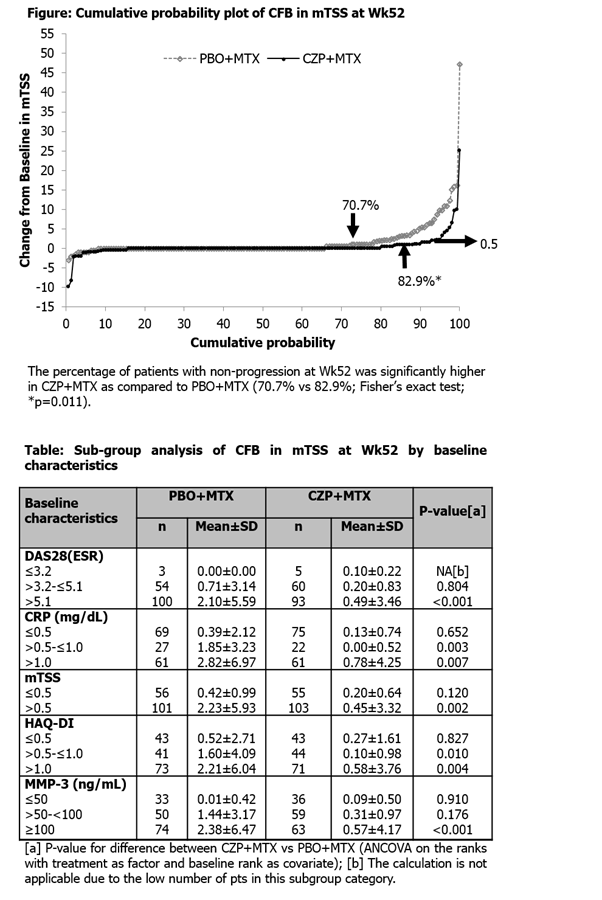
A total of 316 pts (CZP+MTX: n=159, PBO+MTX: n=157) were randomized and received ≥1 dose of study drug. Groups were well-balanced at BL, with mean (±SD) age: 49.2±10.5 yrs, symptom duration: 4.1±2.9 months, DAS28[ESR]: 5.45±1.15 and mTSS: 5.55±12.43. Average MTX dose was 11.6±2.8 mg/wk. CFB in mTSS at Wk52 and 24 for CZP+MTX were, on average, 0.36 and 0.26, and for PBO+MTX were 1.58 and 0.86, respectively (Figure). Statistical comparison of CFB in mTSS by nonparametric approach (ANCOVA on ranks) was significantly greater for CZP+MTX than PBO+MTX at Wk52 (p<0.001) and Wk24 (p=0.003) (Figure). CZP+MTX remission rates were significantly greater than for PBO+MTX at Wk52 (SDAI: 57.9% vs 33.8% [p<0.001]; Boolean: 45.3% vs 28.0% [p=0.002]; DAS28[ESR]: 57.2% vs 36.9% [p<0.001]) and at Wk24 (SDAI: 48.4% vs 29.3% [p<0.001]; Boolean: 36.5% vs 22.3% [p=0.007]; DAS28[ESR]: 52.8% vs 30.6% [p<0.001] Fisher's Exact test). Higher BL DAS28[ESR], CRP, mTSS, HAQ-DI and serum MMP-3 seemed associated with more radiographic progression in the PBO+MTX group, whereas CZP+MTX still inhibited progression of structural damage in these pts despite this higher risk of progression (Table). CZP+MTX was well tolerated, with no unexpected safety signals observed.
Conclusion
CZP+MTX was significantly more effective than PBO+MTX in inhibiting disease progression, as well as in achieving clinical remission, in MTX-naïve Japanese early RA pts with poor prognostic factors.
Reference 1. Aletaha D. Ann Rheum Dis 2010;69:1580–8
Disclosure:
T. Atsumi,
Astellas,Bistol-Myers,Chugai and Mitsubishi-Tanabe ,
8;
K. Yamamoto,
UCB Pharma, Pfizer, Abbott, BMS, Roche, Chugai, Mitsubishi-Tanabe and Eisa,
5,
UCB Pharma, Pfizer, Abbott, Santen, Mitsubishi-Tanabe and Eisai.,
2;
T. Takeuchi,
AstraZeneca, Eli Lilly, Novartis, Mitsubishi-Tanabe and Asahi Kasei,
5,
Abott, Astellas, BMS, Chugai, Daiichi-Sankyo, Eisai, Janssen, Mitsubishi-Tanabe, Nippon Shinyaku, Otsuka, Pfizer, Sanofi-Aventis, Santen, Takeda and Teijin,
2,
UCB Pharma, Abbott, BMS, Chugai, Eisai, Janssen, Mitsubishi-Tanabe, Pfizer and Takeda,
8;
H. Yamanaka,
UCB Pharma, Abbott, Astellas, BMS, Chugai, Eisai, Janssen, Mitsubishi-Tanabe, Pfizer and Takeda,
5,
UCB Pharma, Abbott, Astellas, BMS, Chugai, Eisai, Janssen, Mitsubishi-Tanabe, Pfizer and Takeda,
2;
N. Ishiguro,
Takeda, Mitsubishi-Tanabe, Astellas, Chugai, Abbott, BMS, Eisai, Janssen, Kaken and Pfizer,
2,
Takeda, Mitsubishi-Tanabe, Astellas, Chugai, Abbott, BMS, Eisai, Janssen, Kaken, Pfizer, Taisho-Toyama and Otsuka,
8;
Y. Tanaka,
BMS, MSD, Chugai, Mitsubishi-Tanabe, Astellas, Abbvie, Daiichi-Sankyo,
2,
UCB Pharma, Mitsubishi-Tanabe, Abbott, Abbvie, Eisai, Chugai, Janssen, Pfizer, Takeda, Astellas, Daiichi-Sankyo, GSK, AstraZeneca, Eli Lilly, Quintiles, MSD, Asahi Kasei,
5,
UCB, Mitsubishi-Tanabe, Abbott, Abbvie, Eisai, Chugai, Janssen, Pfizer, Takeda, Astellas, Daiichi-Sankyo, GSK, AstraZeneca, Eli Lilly, Quintiles, MSD, Asahi Kasei,
8;
K. Eguchi,
UCB Pharma,
5;
A. Watanabe,
Daiichi-Sankyo, Kyorin, Shionogi, Taisho, Dainippon-Sumitomo, Taiho, Toyama Chemical and Meiji Seika,
2,
MSD, GSK, Shionogi, Daiichi-Sankyo, Taisho-Toyama, Dainippon-Sumitomo, Mitsubishi-Tanabe, Pfizer,
8;
H. Origasa,
UCB Pharma and Astellas,
5;
T. Shoji,
UCB Pharma,
3;
O. Togo,
UCB Pharma,
3;
T. Okada,
None;
D. M. van der Heijde,
AbbVie, Amgen, AstraZeneca, Augurex, BMS, Celgene, Centocor, Chugai, Covagen, Daiichi, Eli-Lilly, Galapagos, GSK, Janssen Biologics, Merck, Novartis, Novo-Nordisk, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB Pharma, Vertex,
5,
Director of Imaging Rheumatology bv,
3;
N. Miyasaka,
Pfizer, Takeda, Mitsubishi-Tanabe, Chugai, Abbott, Eisai and Astellas,
2;
T. Koike,
Abbvie, Astellas, BMS, Chugai, Daiichi-Sankyo, Eisai, Mitsubishi-Tanabe, Pfizer, Santen, Taisho-Toyama, Takeda, Teijin, UCB Pharma,
5,
UCB Pharma, Pfizer, Chugai, Abbott, Mitsubishi-Tanabe, Takeda, Eisai, Santen, Astellas, Taisho-Toyama, BMS, Teijin and Daiichi-Sankyo,
8.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-first-multicenter-double-blind-randomized-parallel-group-study-of-certolizumab-pegol-in-early-rheumatoid-arthritis-demonstrates-inhibition-of-joint-damage-progression/