Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Telemedicine (TM) has been integral to rheumatology care since the Covid-19 pandemic; evidence suggests high acceptance, satisfaction, and feasibility. However, due to the complexity of SLE outcomes, physicians have raised concerns about the accuracy of TM-derived disease activity (DA) measures. This study aims to evaluate the accuracy of SLE virtual DA measures and whether physician assessments of SLE DA obtained during TM are comparable to those obtained during face-to-face (F2F) visits.
Methods: This is an observational, longitudinal study of SLE patients from 4 academic lupus centers serving diverse populations supported by a grant from the US Department of Defense. Each study participant was evaluated at a baseline and follow-up visit) as dictated by usual care. Virtual physical exam guidelines were established and relied on physician-directed self-examination. At each visit, participants are evaluated by the same provider via a videoconference-based TM and then a F2F encounter. SLE DA measures are completed after the TM and the F2F encounter and the degree of agreement is analyzed using the Bland-Altman method. Tandem provider and patient feedback tools for encounters probe satisfaction, comfort, and problematic evaluation. A planned interim analysis was conducted after the enrollment of the first 50 participants.
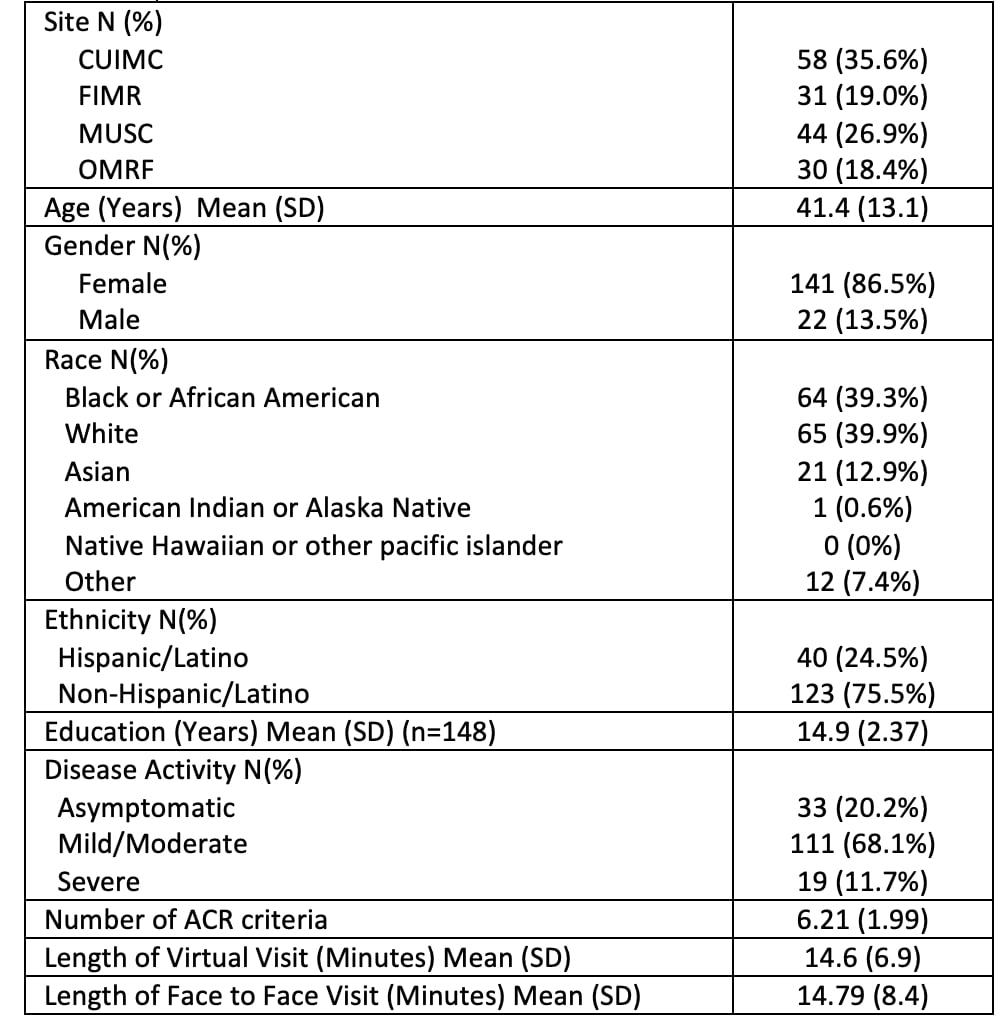
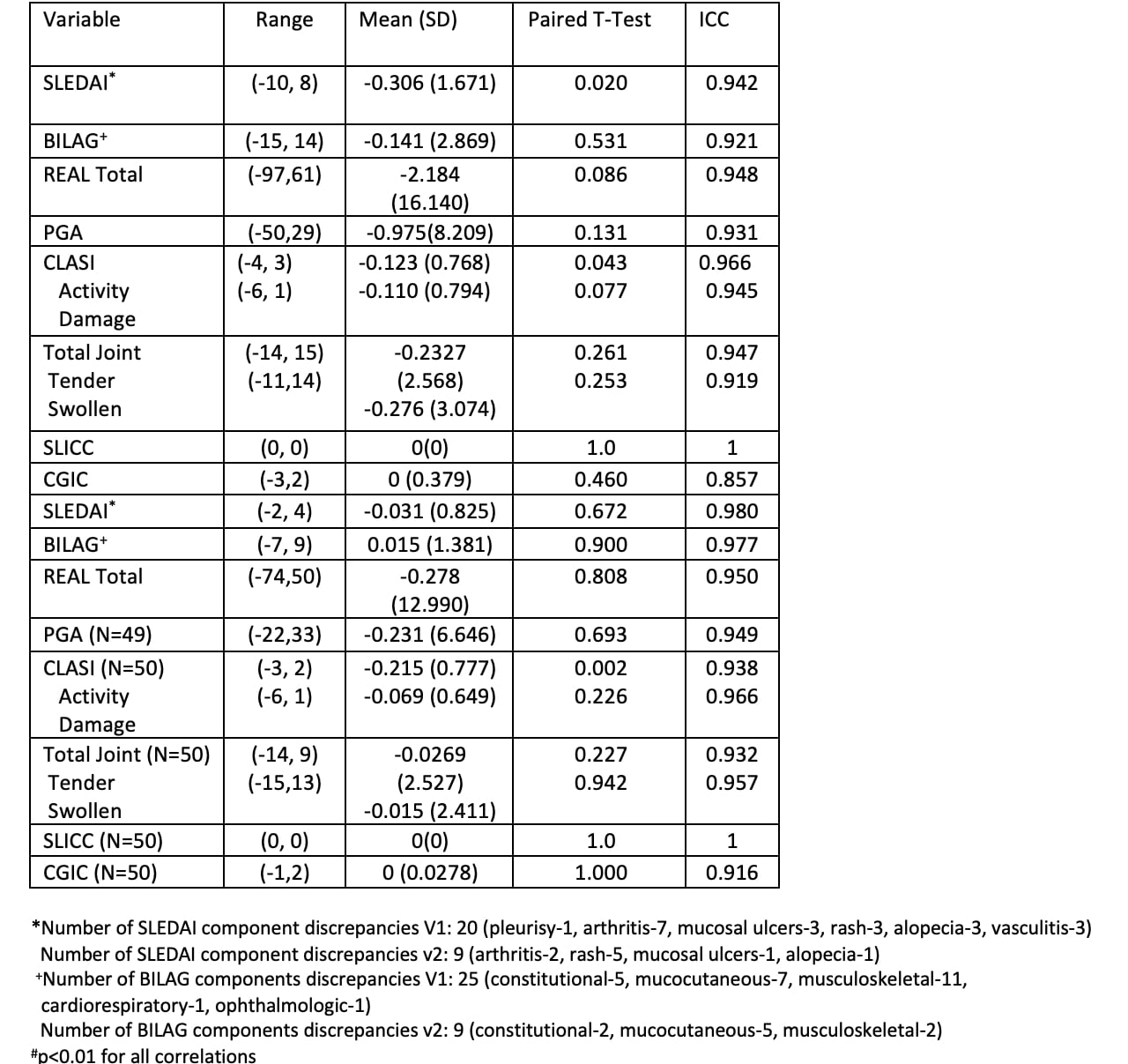
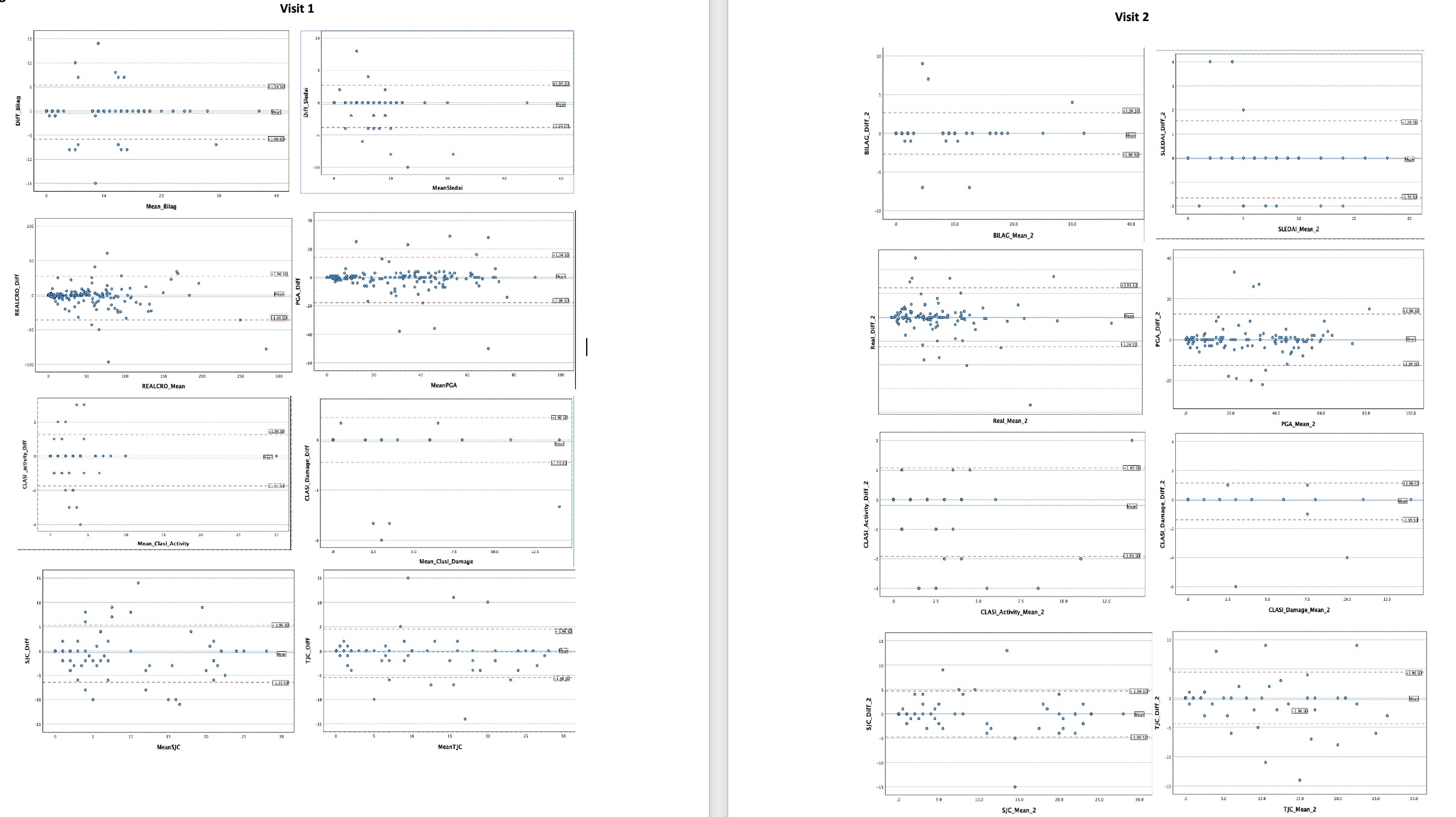
Results: 163 racially and ethnically diverse participants were enrolled, 130 completed the follow-up visit. The baseline characteristics are summarized in Table 1, 87% women, mean age 41.4 ± 13. The enrollment covers a wide range of DA levels (20% inactive, 68% mild/moderate, 12% severe). The differences between DA measures for the visits are summarized in Table 2, TM tended to slightly underestimate DA. Intraclass correlation coefficients (ICC) showed high correlation between TM and F2F at V1 (0.86- 1) and at V2 (0.92 – 1). There were 29 SLEDAI discrepancies (pleurisy -1, arthritis-9, mucosal ulcers-4, rash-8, alopecia-4, vasculitis-3) and 34 BILAG discrepancies (constitutional-7, mucocutaneous-12, musculoskeletal-13, cardiorespiratory-1, ophthalmologic-1). The Bland Altman plots of TM-F2F differences and scatter plots are shown in Figure 1. Paired T test showed significant differences in CLASI activity scores between TM and F2F visits for V2. Physicians reported high levels of satisfaction (highly satisfied or satisfied) for 88.5% of total TM visits. Following the F2F encounter, the evaluators confirmed that they considered their virtual encounter assessments to be accurate for 95.5% of visits.
Conclusion: This data shows high correlations between virtual and F2F DA measures. Mucocutaneous and arthritis domains had the most differences. The current study evaluating TM-derived outcome measures in SLE provides quantitative and qualitative data on the comparability between virtual and F2F DA measures. This data supports an increase in the use of TM in both lupus clinical care and research. All changes in DA- flares observed in a TM encounter should trigger a F2F evaluation as TM visits can sometimes overestimate disease activity.
To cite this abstract in AMA style:
Souvignier M, Aranow C, Askanase A, Kim M, Kamen D, Arriens C, Khalili L, Tang W, Dall'Era M, Mackay M. The Evaluation of SLE Outcome Measures in Telemedicine [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-evaluation-of-sle-outcome-measures-in-telemedicine/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-evaluation-of-sle-outcome-measures-in-telemedicine/