Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient reported outcome measures (PROM) are required as key outcomes in disease modifying therapeutic trials in systemic sclerosis (SSc). A PROM tool in SSc, covering the different features of this multi-organ autoimmune disease, is lacking. In this study, we aim to develop and validate a brief, disease-specific, patient-derived, composite disease impact score for scientific and clinical use in SSc.
Methods: This multi-center project endorsed by EULAR involves SSc patients and experts from 11 European countries. Firstly, using the nominal group technique, patients with SSc selected the health dimensions where the disease has the most significant impact. The dimensions were subsequently given numeric priority by an international group of SSc patients. Patients were asked to rank the dimensions in order of their importance by giving a rank from 1(most important) to 17 (least important). Each rank could only be used once. The dimensions with the top 10 median ranks, as ranked by the patients, were used to construct the ScleroID questionnaire using numeric rating scales. An observational study to weight the dimensions in order to calculate the ScleroID score and to validate the questionnaire is currently ongoing and aims to target a larger cohort of patients from all participating countries.
Results: 24 SSc patients selected 17 health dimensions in the nominal group exercise. The prioritization cohort included 108 SSc patients from 11 centers (female:male 82:25, limited: diffuse SSc subset 53:54). The top 10 health dimensions which were ranked most relevant by the patients were Raynaud’s phenomenon, hand function, upper and lower gastrointestinal tract symptoms, pain, fatigue, limitation of life choices and activities, body mobility, breathlessness and digital ulcers. Based on this, the ScleroID questionnaire was constructed (Figure 1).
Conclusion: The EULAR ScleroID score is a novel, patient-derived, tool under development designed for use in clinical practice and clinical trials to display the disease impact of SSc. A large scale observational study for weighting of the dimensions and validation of this new instrument is ongoing. Figure 1. The ScleroID questionnaire.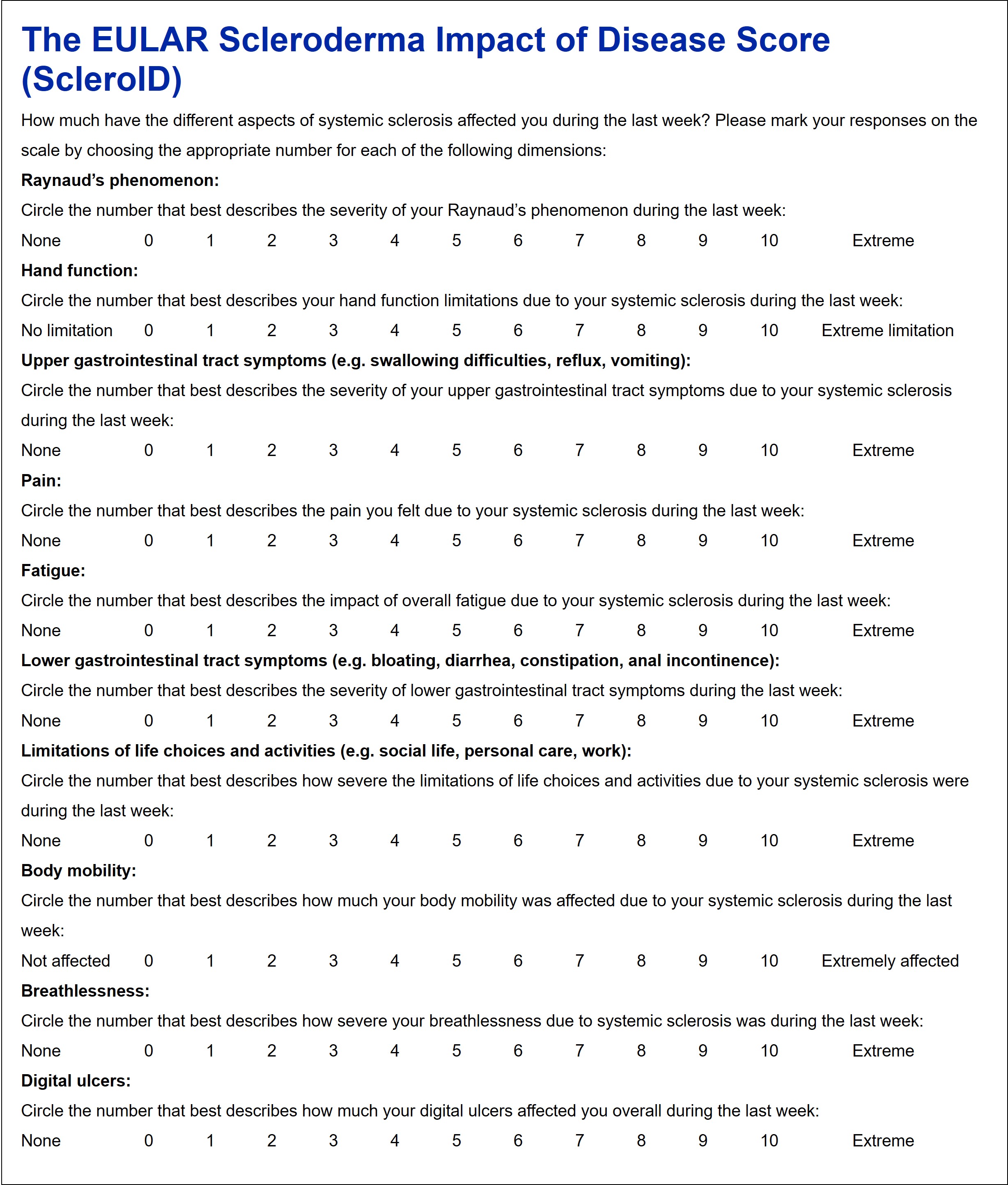
To cite this abstract in AMA style:
Dobrota R, Becker M, Fligelstone K, Fransen J, Kennedy A, Allanore Y, Carreira P, Czirják L, Denton C, Hesselstrand R, Sandqvist G, Kowal-Bielecka O, Matucci Cerinic M, Mihai C, Gheorghiu AM, Müller-Ladner U, Frerix M, Heiberg T, Distler O. The EULAR Systemic Sclerosis Impact of Disease Score – a New Patient-Reported Outcome Measure for Patients with Systemic Sclerosis Under Development [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/the-eular-systemic-sclerosis-impact-of-disease-score-a-new-patient-reported-outcome-measure-for-patients-with-systemic-sclerosis-under-development/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-eular-systemic-sclerosis-impact-of-disease-score-a-new-patient-reported-outcome-measure-for-patients-with-systemic-sclerosis-under-development/
