Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Pregabalin has demonstrated efficacy for the treatment of fibromyalgia (FM), but insufficient evidence exists on how the efficacy of pregabalin may differ by baseline pain severity. The objective of these analyses was to assess the efficacy of 12 weeks of pregabalin treatment to provide symptomatic pain relief in FM patients with moderate or severe baseline pain scores.
Methods: These analyses used data from 5 Phase III clinical trials ranging between 8–15 weeks of pregabalin versus placebo (study numbers 1008105, A0081056, A0081077, A0081100, A0081208) to evaluate the efficacy of pregabalin at doses of 300 mg/day, 450 mg/day, or flexible dosing (300-450 mg/day BID) for the treatment of FM. FM was defined by ACR 1990 criteria at screening of widespread pain ≥3 months and pain in ≥11 of 18 specific tender point sites. Patients were adult (aged ≥18 years), with mean baseline pain scores that were classified as moderate (≥4-<7) or severe (≥7-10) and a score ≥40 mm on the Visual Analog Scale of Short-form McGill Pain Questionnaire. A mixed effects model repeated measures analysis was used to estimate change in pain at 12 weeks after treatment initiation by baseline severity (moderate or severe) and treatment.
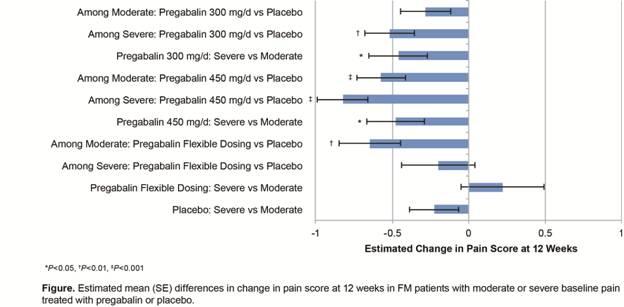
Results: Overall, 679 (300 mg/d pregabalin), 670 (450 mg/d pregabalin), 250 (flexible dosing pregabalin), and 927 (placebo) patients were evaluated. The results of change in pain score at 12 weeks are displayed in the figure.
Among patients who had moderate baseline pain, significantly larger mean ± SE reductions in pain score were observed with pregabalin versus placebo at doses of 450 mg/d (-0.572 ± 0.161, P<0.001) and flexible dosing (-0.647 ± 0.203, P=0.002) with a non-significant reduction at the 300 mg/d dose (-0.282 ± 0.165). Patients with severe baseline pain showed significant improvements over placebo with pregabalin doses of 300 mg/d (-0.516 ± 0.164, P=0.002) and 450 mg/d (-0.822 ± 0.165, P<0.001), but not with flexible dosing (-0.200 ± 0.242). When patients were grouped by pregabalin dose assignment, patients with severe baseline pain exhibited greater improvements in pain score than patients with moderate baseline pain with 300 mg/day (-0.461 ± 0.191, P=0.016) and 450 mg/day doses (-0.476 ± 0.189, P=0.012), but not with flexible dosing (+0.220 ± 0.272).
Conclusion: Pregabalin is efficacious at 12 weeks of treatment in reducing pain in FM patients with baseline moderate pain and severe pain severity, with reductions that appear larger in patients with severe pain. The trend of larger changes following flexible dosing in patients with moderate versus severe baseline pain scores comes from a study of Japanese patients and additional research would be required to determine if this may be accounted for by differences in the average daily dose.
Disclosure:
A. Clair,
Pfizer Inc,
3,
Pfizer Inc,
1;
B. Emir,
Pfizer Inc.,
1,
Pfizer Inc.,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-efficacy-of-pregabalin-for-treating-fibromyalgia-patients-with-moderate-or-severe-baseline-widespread-pain/