Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: RA patients (pts) are at increased risk of herpes zoster (HZ), and ACR guidelines recommend vaccination in pts aged ≥50 years prior to starting biologic DMARDs or tofacitinib.1 Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. Live zoster vaccine (LZV) has shown 70% efficacy in immunocompetent adults aged 50–59 years, and 51% efficacy in those aged ≥60 years.2 We previously reported that RA pts on background methotrexate who started 3 months of treatment with tofacitinib after LZV had similar varicella zoster virus (VZV)-specific immunity to placebo (PBO) pts, and that their VZV immunity at Week (Wk) 6 post-vaccination was comparable with healthy individuals aged ≥50 years.3 The objective of this study was to evaluate the long-term effectiveness of LZV in pts with RA, via the incidence of HZ after treatment with tofacitinib for up to 27 months.
Methods: The initial study involved 112 RA pts given LZV and randomized 2–3 wks later to tofacitinib 5 mg twice daily (BID) or PBO for 12 wks (A3921237 [NCT02147587]). At 14 wks post-vaccination, pts joining the long-term extension ORAL Sequel study (NCT00413699) initiated open-label treatment with tofacitinib 5 or 10 mg BID. The incidence of post-vaccination HZ after tofacitinib exposure up to 27 months (based on an extended follow-up beyond the January 2016 data snapshot) was evaluated. Among HZ cases, we analyzed measures of VZV-specific immunity with average immunity after LZV.
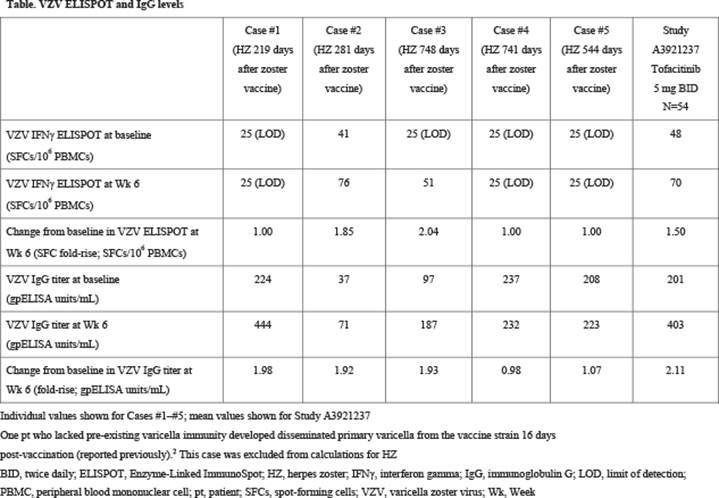
Results: 112 pts were randomized to PBO (n=57) or tofacitinib 5 mg BID (n=55). 100 pts continued to receive tofacitinib in ORAL Sequel. Five cases (not adjudicated) of HZ occurred, with an incidence rate (pts with events per 100 pt-years) of 3.60 (95% confidence interval 1.17, 8.39) (#1: 202 days [219 days post-LZV], #2: 267 days [281], #3: 702 days [748], #4: 699 days [741], #5: 446 days [544] after initiation of tofacitinib). Cases #1–#4 were monodermatomal; case #5 involved 5 dermatomes. All cases resolved with treatment. Cases #1, #4, and #5 had undetectable ELISPOT measures at baseline and at Wk 6 post-vaccination, indicating a lack of VZV-specific immunity. Cases #2 and #3 responded adequately to vaccination by both immunoglobulin G (IgG) and ELISPOT measures, but had lower-than-average VZV IgG levels, both at baseline and at Wk 6 (Table).
Conclusion: LZV prior to treatment with tofacitinib is effective at boosting IgG levels and cell-mediated immunity towards VZV. Of the 5 pts who developed HZ, 3 did not have any measurable cell-mediated response, and 2 had a low humoral response.
References: 1. Singh JA et al. Arthritis Care Res (Hoboken) 2016;68:1-25.
2. Hales CM et al. MMWR Morb Mortal Wkly Rep 2014;63:729-31.
3. Winthrop K et al. Arthritis Rheumatol 2015;67:Abstract 12L.
Acknowledgment: The authors would like to acknowledge Lisa McNeil.
To cite this abstract in AMA style:
Winthrop K, Wouters A, Choy E, Nduaka C, Biswas P, Wang L, Hodge J, Lazariciu I, Soma K, Mojcik CF, Needle E, Rigby WFC. The Effectiveness of Zoster Vaccine in RA Patients Subsequently Treated with Tofacitinib [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/the-effectiveness-of-zoster-vaccine-in-ra-patients-subsequently-treated-with-tofacitinib/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effectiveness-of-zoster-vaccine-in-ra-patients-subsequently-treated-with-tofacitinib/