Session Information
Date: Monday, November 6, 2017
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy I: Outcomes Therapy
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: IL-6 involvement has been reported in glucose metabolism.1-4 Sarilumab, a human mAb blocking the IL-6Rɑ, was evaluated for treatment of RA in 3 clinical trials: MOBILITY, TARGET, and MONARCH. This analysis examined effects of sarilumab or placebo (Pbo), plus DMARDs, on fasting glucose and glycosylated hemoglobin (HbA1c) in patients with RA, with and without diabetes.
Methods: Fasting glucose and HbA1c data were collected during 2 Pbo-controlled studies of sarilumab + DMARDs: MOBILITY and TARGET. Studies excluded patients with HbA1c ≥9.0%. Patients with baseline (BL) and ≥1 post-BL sample were included in post hoc pooled analyses and categorized as diabetic (DIAB) or non-DIAB based on medical history of diabetes or prior use of antidiabetic medication. Changes from BL in fasting glucose, HbA1c, and weight were analyzed with a linear regression model. Spearman rank correlation coefficient was calculated for changes in glucose, HbA1c, and high-sensitivity (hs)-CRP. Changes in these parameters were stratified by clinical response. Clinical meaningfulness was based on comparison with results of clinical trials with antidiabetic medications.5
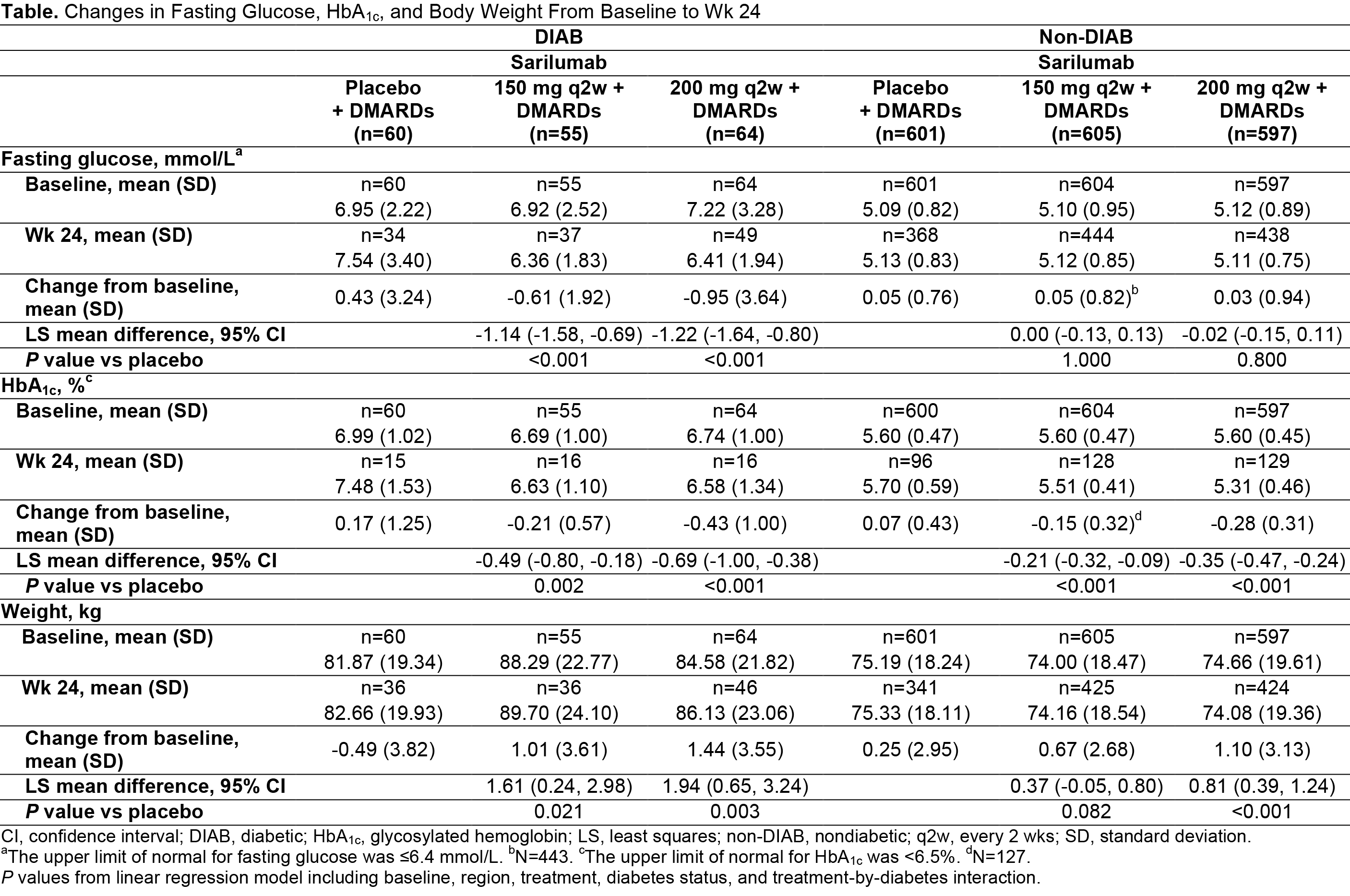
Results: The DIAB group (n=179) compared with the non-DIAB group (n=1803) had higher BL body weight (mean ± SD, kg: 84.8 ± 21.4 vs 74.6 ± 18.8) and a larger proportion of patients with BMI ≥30 kg/m2 (56.7% vs 31.9%). Mean fasting glucose and HbA1c at BL were similar across treatment groups (Pbo, and sarilumab 150 and 200 mg every 2 wks [q2w]) but higher in the DIAB group than in the non-DIAB group (Table). Patients in the DIAB group had a greater reduction in fasting glucose at wk 24 compared with those in the non-DIAB group. Decreases in HbA1c occurred in non-DIAB and DIAB sarilumab-treated groups but not with Pbo. The treatment effect was largest in the DIAB group. At wk 24, the change in HbA1c was -0.43% in the DIAB (sarilumab 200 mg q2w) group and +0.17% in the DIAB (Pbo) group (-0.69% mean difference; P<0.001). Reductions in fasting glucose and HbA1c were observed in sarilumab-treated DIAB subgroups independently of changes in hs-CRP, ACR50, or DAS28-CRP remission status, and despite increases in mean body weight. The overall safety of sarilumab did not differ between DIAB and non-DIAB patients with RA.
Conclusion: Sarilumab + DMARDs reduced fasting glucose and HbA1c in DIAB and HbA1c in non-DIAB patients with RA, independent of changes in body weight, hs-CRP, ACR50, or DAS28-CRP remission status at wk 24. In DIAB patients, the difference in HbA1c reduction between sarilumab 200 mg q2w and Pbo was clinically meaningful.
References:
1. Kristiansen et al. Diabetes. 2005;54(suppl 2):S114-S124.
2. Ellingsgaard et al. Nat Med. 2011;17:1481-1489.
3. Ogata et al. Ann Rheum Dis. 2011;70:1164-1165.
4. Schultz et al. PLoS One. 2010;5:e14328.
5. Bolen et al. Diabetes Medications for Adults With Type 2 Diabetes: An Update. Comparative Effectiveness Review No. 173. 2016.
To cite this abstract in AMA style:
Genovese MC, Fleischmann R, Hagino O, Hu CC, Pena-Rossi C, Sadeh J, Graham NMH, Mangan EK, van Hoogstraten H, Mandrup-Poulsen T. The Effect of Sarilumab in Combination with Dmards on Fasting Glucose and Glycosylated Hemoglobin in Patients with Rheumatoid Arthritis with and without Diabetes [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/the-effect-of-sarilumab-in-combination-with-dmards-on-fasting-glucose-and-glycosylated-hemoglobin-in-patients-with-rheumatoid-arthritis-with-and-without-diabetes/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-sarilumab-in-combination-with-dmards-on-fasting-glucose-and-glycosylated-hemoglobin-in-patients-with-rheumatoid-arthritis-with-and-without-diabetes/