Session Information
Date: Monday, October 22, 2018
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Adult onset Still¡¯s disease (AOSD) is a rare inflammatory disorder of unknown etiology. Macrophage activation syndrome (MAS) is a life-threatening complication and has been recognized to complicate AOSD. Etoposide(VP-16) has been previously utilized in patients with MAS and induced a favorable and rapid response in cases. In this study, we conducted a single-center, retrospective survey to evaluate the efficacy of etoposide in inducing remission in a series of refractory AOSD patients.
Methods: We included 42 refractory AOSD patients who previously received high dose of steroids (¡Ý2 mg/kg/day of prednisone), immunosuppressive drugs and biologic agents. HLH-2004 was used to define AOSD-MAS, and sJIA MAS-2016 was used to enclose those with MAS tendencies(pre-MAS). 23 patients were treated with etoposide(VP-16 group) and 19 patients were treated with methotrexate(MTX) or cyclosporine(CsA)(Control Group). We evaluated disease course, efficacy of treatment and potential adverse effects for at least one year. Efficacy was evaluated as ¡®Partial response¡¯ (PR: clinical improvement without normalization of inflammatory markers, nor >50% reduction in the dose of prednisone).
Results: The average age was 36¡À14 years in VP-16 group and 37¡À13 years in control group. MAS and Pre-MAS of each group were observed in 48% vs.11% and 47% vs. 42%. At the time of VP-16/Control treatment, the most frequent clinical manifestation was fever (100%/100%), Rash (87%/100%) and Joints involvement (78%/79%). The laboratory parameters such as ferritin, triglycerides and alanine transaminase showed higher level in VP-16 group at baseline.
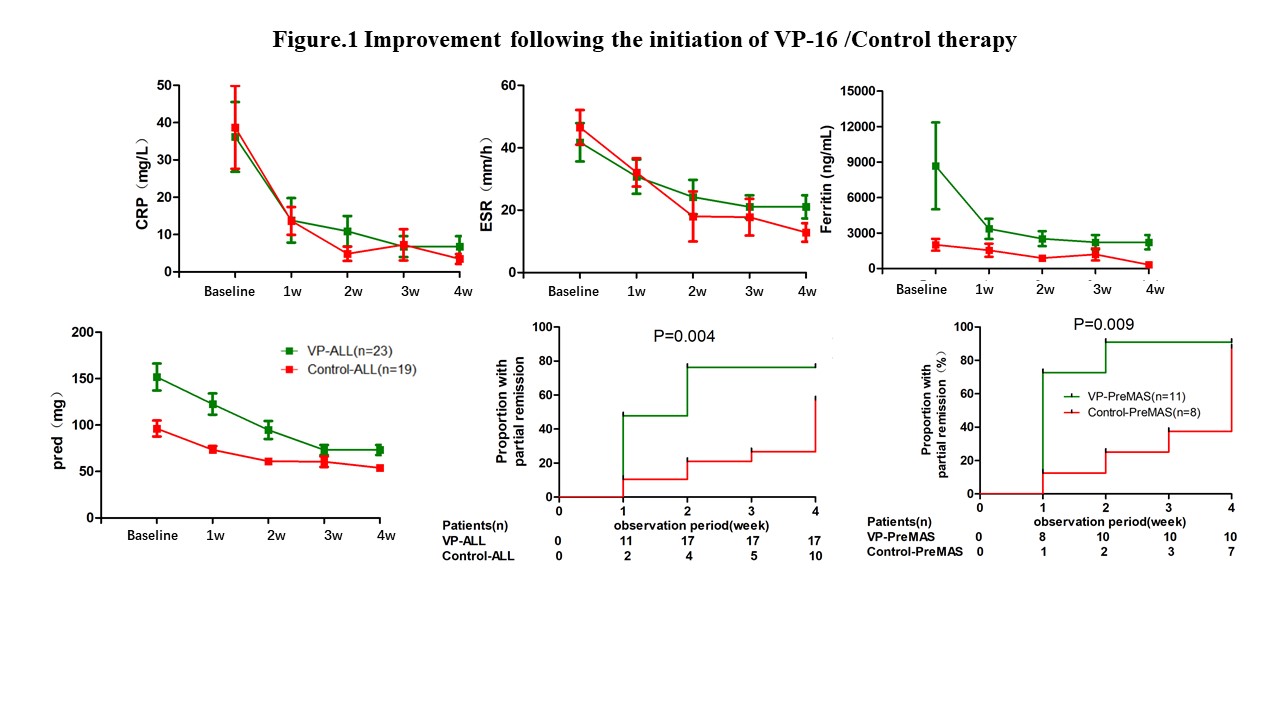
The median dosages of VP-16 were 575mg (IQR 150-1400mg). The median treatment course were 4 weeks (IQR 2 weeks-10 months). The majority of patients showed clinical improvement after VP-16/Control therapy. A dramatic reduction of inflammatory laboratory markers and prednisone dose were achieved. 17 patients responded to VP-16 (PR 74%) and 10 patients responded to CsA or MTX (PR 53%) at 4th week. Besides, in Pre-MAS patients, compared with CsA or MTX, there was rapid increase of PR in VP group(Figure 1).
Adverse events were seen as follows: hemocytopenia (n=2/n=1), gastrointestinal effects (n=3/n=1), alopecia (n=1/n=0), and infections (n=6/n=5). 2 patients died (one with shock and one with infection) in VP-16 group and the rest survived. With an average follow-up of 21¡À11 months/24¡À14 months, a decrease in the dose of steroids and immunosuppressants was possible in all patients [discontinuation: 9%/16%; steroid monotherapy (¡Ü15mg/d): 13%/5%; steroids + DMARDs: 57%/42%].
Conclusion: Etoposide treatment was associated with rapid and maintained clinical and laboratory improvement in patients with AOSD refractory. It is necessary to carry out large samples and long-term follow-up clinical studies to evaluate its exact effects and safety.
To cite this abstract in AMA style:
Wang H, Wang X, Li T, Ye S. The Effect of Etoposide on Inducing Remission in Refractory Adult-Onset Still’s Disease: A Retrospective, Single-Center Study [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/the-effect-of-etoposide-on-inducing-remission-in-refractory-adult-onset-stills-disease-a-retrospective-single-center-study/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-etoposide-on-inducing-remission-in-refractory-adult-onset-stills-disease-a-retrospective-single-center-study/