Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Tumor necrosis factor inhibitors (TNFi) should be used for the treatment of rheumatoid arthritis (RA) in combination with conventional synthetic disease modifying anti‑rheumatic drugs (csDMARD), preferably with methotrexate (MTX). However a significant proportion of RA patients receive TNFi in combination with other csDMARD or in monotherapy. We aimed to assess the effect of co-medication with MTX or other csDMARDs on drug survival of the first TNFi in RA.
Methods: All adult patients with RA followed in the Czech national registry ATTRA who started TNFi therapy after January 1st 2012 were considered. Baseline demographic data of patients starting their first TNFi in combination with MTX, with other csDMARD or as a monotherapy were compared. Six‑year drug survival was analyzed using Kaplan-Meier method, log rank test was used to compare differences between groups. ATTRA is a centralized prospective computerized registry collecting data on efficacy, safety and quality of life of patients treated with biologic disease modifying anti-rheumatic drugs (bDMARD) in the Czech Republic. TNFi therapy is usually indicated for patients with RA who have failed treatment with at least one csDMARD.
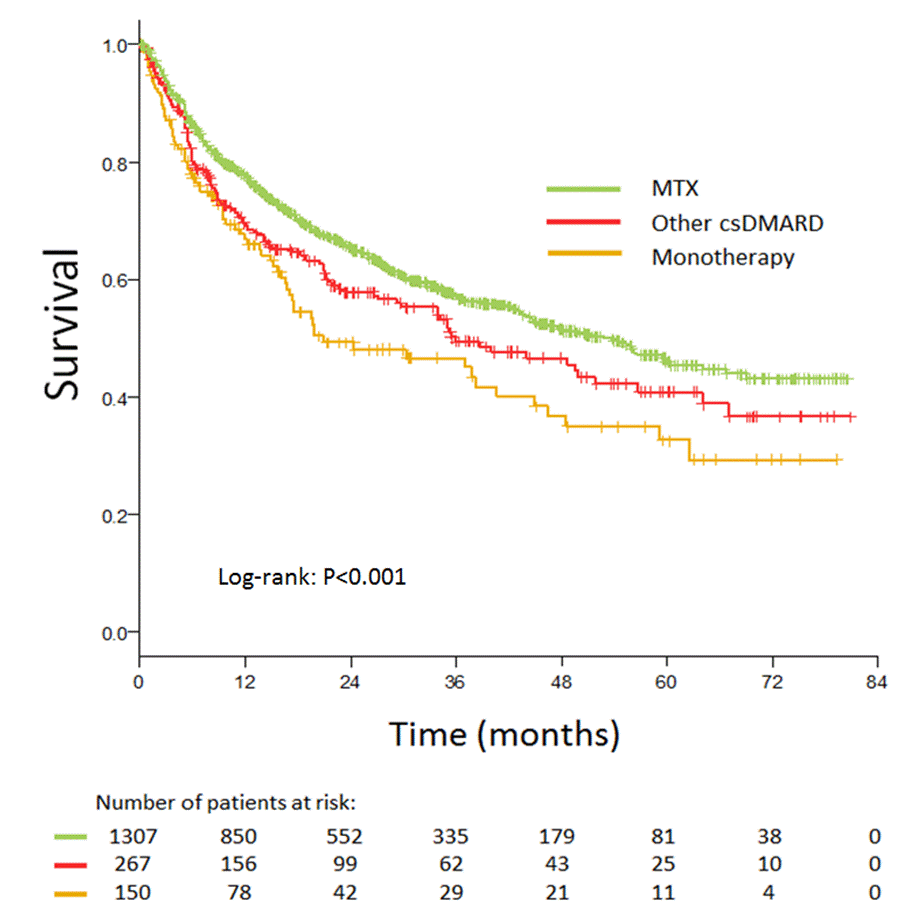
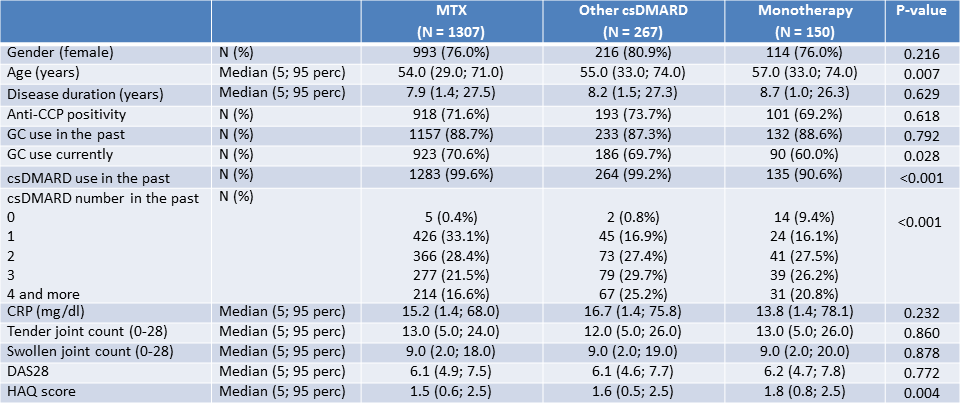
Results: A total of 1841 RA patients initiated first bDMARD treatment during the studied period, with 1724 patients receiving TNFi. 1307 patients (76%) started TNFi therapy in combination with MTX (median dose 15mg weekly), 267 patients (15%) with other csDMARD (most commonly leflunomide) and 150 patients (9%) as monotherapy. The baseline disease activity based on the number of tender/swollen joints, CRP levels and DAS-28 scores was comparable between the three groups. There was a significantly higher number of patients without history of csDMARD therapy prior to TNFi initiation in the monotherapy group (Table 1). Overall TNFi drug survival was significantly better in patients receiving MTX co-medication (median survival 53 months) compared to those on monotherapy (median survival 21 months). Patients receiving TNFi in combination with other csDMARD had median survival worse than those on methotrexate and better than those on monotherapy (median survival 36 months), albeit the difference was not statistically significant (Figure 1). The most common reason for TNFi discontinuation was loss of efficacy (33%, 37% and 28% for MTX, other csDMARD combination and monotherapy respectively) followed by primary inefficacy (20%, 19% and 27%) and adverse effects (19%, 15% and 23%).
Conclusion: In this registry study of patients with RA, use of MTX co-medication was associated with significantly better first TNFi drug survival compared to monotherapy.
Disclaimer: This study was supported by the project of MHCR for conceptual development of research organization 00023728
To cite this abstract in AMA style:
Mann H, Nekvindová L, Závada J, Křístková Z, Horák P, Vencovský J, Pavelka K. The Effect of Co-medication with Methotrexate and Other Conventional Synthetic Disease Modifying Anti-rheumatic Drugs on First Tumor Necrosis Inhibitor Drug Survival in Patients with Rheumatoid Arthritis: Results Form a Nationwide Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-effect-of-co-medication-with-methotrexate-and-other-conventional-synthetic-disease-modifying-anti-rheumatic-drugs-on-first-tumor-necrosis-inhibitor-drug-survival-in-patients-with-rheumatoid-arthri/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-co-medication-with-methotrexate-and-other-conventional-synthetic-disease-modifying-anti-rheumatic-drugs-on-first-tumor-necrosis-inhibitor-drug-survival-in-patients-with-rheumatoid-arthri/