Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
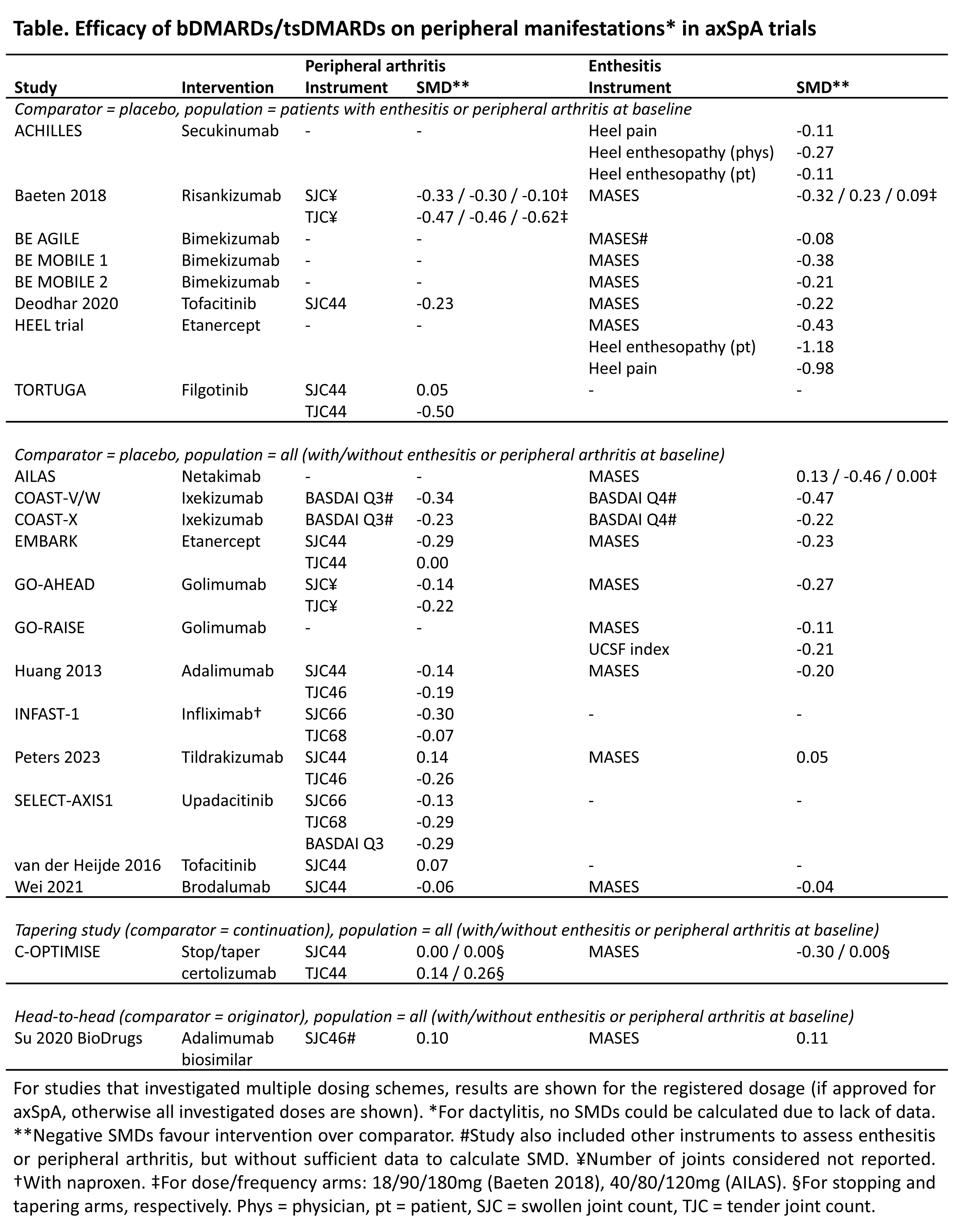
Background/Purpose: In axial spondyloarthritis (axSpA), peripheral musculoskeletal manifestations (peripheral arthritis, enthesitis, dactylitis) are common and contribute to the burden of disease. Our objective was to evaluate the evidence on the efficacy of biological and targeted synthetic therapies (bDMARDs/tsDMARDs) on peripheral musculoskeletal manifestations in axSpA.
Methods: Systematic literature review (SLR) of controlled trials that assessed the effect of bDMARDs/tsDMARDs compared to any active treatment or placebo on peripheral arthritis, enthesitis or dactylitis in adult axSpA patients (PROSPERO CRD42024532666). Studies were identified using previous SLRs on bDMARD/tsDMARD efficacy for the 2016/2022 updates of the ASAS-EULAR axSpA management recommendations (≤2021)1-3 and an updated search (2022 – March 2024). Standardised mean differences (SMDs; < 0.5 small, 0.5-0.8 moderate, >0.8 large effect; negative SMDs favour intervention) were calculated for change from baseline, and complete resolution of each peripheral manifestation was assessed. If SMDs could not be calculated, post-intervention outcome values were considered instead.
Results: In total, 50 studies were included. Most investigated bDMARDs, especially TNF inhibitors (n=24 [48%]) and IL-17 inhibitors (n=13 [26%]), while few focused on tsDMARDs (n=6 [12%]). Studies often compared active treatment to placebo (n=35 [70%]), although several included a head-to-head comparison (n=6 [13%], usually biosimilar versus originator comparison). Some studies (n=4 [8%]) focused on tapering or complete withdrawal of bDMARDs. Risk of bias was often low (n=33 [66%]).Of the 50 included studies, 37 (74%) reported results on peripheral arthritis, 46 (92%) on enthesitis and only 1 (2%) on dactylitis. Effects on peripheral arthritis were small for both bDMARDs and tsDMARDs (n=16 studies), with most SMDs ranging from -0.5 to 0 (Table). Of note, only few studies reported results in the subgroup with peripheral arthritis at baseline, showing slightly higher effects. Complete resolution of peripheral arthritis in those affected at baseline (n=2 studies, both on bimekizumab) occurred in 58-64% for bimekizumab and 36-42% for placebo. Similar to peripheral arthritis, SMDs indicated mainly small to moderate effects on enthesitis for both bDMARDs and tsDMARDs (n=19 studies, Table). Complete resolution of enthesitis (n=5 studies) occurred in 34-52% of patients for various bDMARDs and 14-33% for placebo. Results were similar in studies for which no SMD could be calculated. Finally, for dactylitis, the only available study was a TNF inhibitor tapering study. Dactylitis counts were similar in both study arms (median of 0 in both), although most patients did not have active dactylitis at baseline.
Conclusion: In axSpA, bDMARDs and tsDMARDs have small to moderate effects on peripheral arthritis and enthesitis. For dactylitis, efficacy data for these drugs are very scarce in axSpA. Most studies do not report results in the population of interest (those affected at baseline), possibly resulting in an underestimation of intervention effects.References1. Sepriano et al. RMD Open 2017.2. Webers et al. ARD 2023.3. Ortolan et al. ARD 2023.
To cite this abstract in AMA style:
Webers C, ORTOLAN A, Nikiphorou E, Sepriano A, Falzon L, López Medina C, capelusnik D, Van Der Heijde D, Molto A, Ramiro S. The effect of biological and targeted synthetic DMARDs on peripheral manifestations in axial spondyloarthritis: a systematic literature review [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/the-effect-of-biological-and-targeted-synthetic-dmards-on-peripheral-manifestations-in-axial-spondyloarthritis-a-systematic-literature-review/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-biological-and-targeted-synthetic-dmards-on-peripheral-manifestations-in-axial-spondyloarthritis-a-systematic-literature-review/