Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The treat-to-target concept is currently recommended in Axial Spondyloarthritis (axSpA) and remission is the main objective of treatment. Although consensual definitions of remission are lacking, ASAS-Partial Remission (ASAS-PR) and ASDAS-Inactive Disease (ASDAS-ID) have gained wide acceptance as clinical remission-like definitions in current practice.
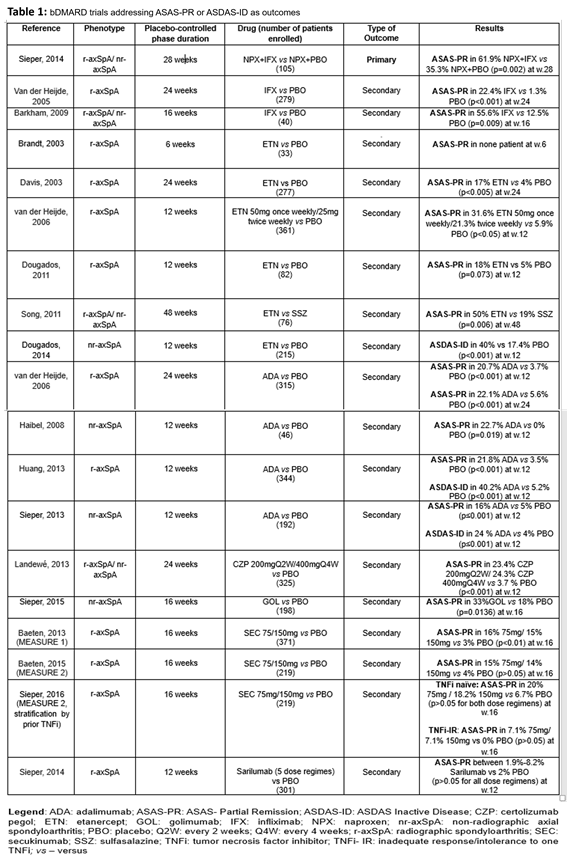
Methods: In this review we assessed the efficacy of different biologic disease-modifying anti-rheumatic drugs (bDMARD) in achieving ASAS-PR or/and ASDAS-ID, as remission-like outcomes. Data from placebo-controlled phases of randomised controlled trials (RCTs) were included. A systematic literature review was performed using the MEDLINE database (May 1 2018) with the filters “published since the year 2000” and “humans”. The PICO (P, population; I, intervention; C, comparison; O, outcome) concept was used to perform the analysis according to: Patients – adults (>18 years old) with radiographic axSpA (r-axSpA) or non-radiographic axSpA (nr-axSpA); Intervention – any bDMARD regardless of formulation or duration; Comparison – placebo and/or any different drug; Outcomes: ASAS-PR and ASDAS-ID. For each outcome, we assessed whether the treatment group was superior, equal or inferior to the comparator group regarding the achievement of the ASAS-PR or ASDAS-ID, as primary outcomes.
Results: After screening 152 references, 19 RCTs fulfilled the inclusion criteria – 15 concerning tumor necrosis factor inhibitors (TNFi), 3 secukinumab (anti-IL17A) and 1 sarilumab (anti-IL6R).
Eleven studies were conducted in r-axSpA, four in nr-axSpA and four in both populations. ASAS-PR was the dominant remission-like definition, used in 18 of the trials. Only 1 RCT used these remission-like endpoints as primary outcomes, in the remaining 18 trials, ASAS-PR or ASDAS-ID were just focused as secondary measures (Table 1). Concerning TNFi bDMARDs, 14 of the 15 trials provide evidence of efficacy in achieving remission, with the proportion of patients achieving ASAS-PR and ASDAS-ID varying between 16-61.9% and 24-40.2% respectively with a minimum and maximum of placebo-controlled phase duration of 12 to 48 weeks. Secukinumab was effective in achieving ASAS-PR when an initial intravenous loading dose was applied (MEASURE 1), while sarilumab was not able to induce remission in axSpA.
Conclusion: Clinical trials addressing remission-like concepts as outcomes are limited. Most studies were conducted in r-axSpA and ASAS-PR score was the preferred remission outcome. Considering nowadays-aimed treatment targets, these data raise the unmet need for improved treatment options favoring optimized remissions rates in axSpA patients.
To cite this abstract in AMA style:
Machado AR, Rodrigues Manica S, Leite Silva J, Pimentel-Santos F, Tavares Costa J, Vieira-Sousa E. The Effect of Biologic Disease-Modifying Antirheumatic Drugs in Targeting Disease Remission in Axial Spondyloarthritis: A Systematic Literature Review [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/the-effect-of-biologic-disease-modifying-antirheumatic-drugs-in-targeting-disease-remission-in-axial-spondyloarthritis-a-systematic-literature-review/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-biologic-disease-modifying-antirheumatic-drugs-in-targeting-disease-remission-in-axial-spondyloarthritis-a-systematic-literature-review/