Session Information
Date: Wednesday, October 24, 2018
Title: 6W027 ACR/ARHP Abstract: Health Services Research II: Economic & Clinical Implications (2994–2999)
Session Type: ACR/ARHP Combined Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Estimates of the economic burden of SLE within economically disadvantaged populations in the US are limited. The purpose of this study is to provide an assessment of the economic burden of SLE in a Medicaid cohort, with an emphasis on disease severity as measured by treatment indicative of moderate-to-severe SLE.
Methods: SLE patients ages 18-64 years treated with antimalarials, immunosuppressants, biologics (belimumab, abatacept, or rituximab), or systemic glucocorticoids from 1/1/2010 through 12/31/2014 were identified from administrative claims in the MarketScan© Medicaid Multi-state database. The first prescription fill date was the index date. Patients were required to have at ≥ 1 inpatient claim or 2 non-diagnostic outpatient claims >30 days apart for SLE during the 12 months prior to the index. If the patient had only outpatient claims, ≥ 1 SLE diagnoses must have been made by a rheumatologist or nephrologist. Patients were categorized according to their SLE treatment during a 6-month exposure period starting on the index as follows: 1) mild SLE: either antimalarial monotherapy or low-dose oral glucocorticoid (<=5 mg/day) monotherapy or 2) moderate-to-severe SLE: any immunosuppressive or combinations of SLE medications other than either antimalarial or low-dose oral glucocorticoid monotherapy. All-cause healthcare utilization and costs were evaluated during the 12 months following the initial exposure period (in 2016 US dollars). Generalized linear modeling with log link and gamma error distribution estimated total costs and total costs excluding outpatient pharmacy costs during follow-up, adjusting for demographic and clinical characteristics.
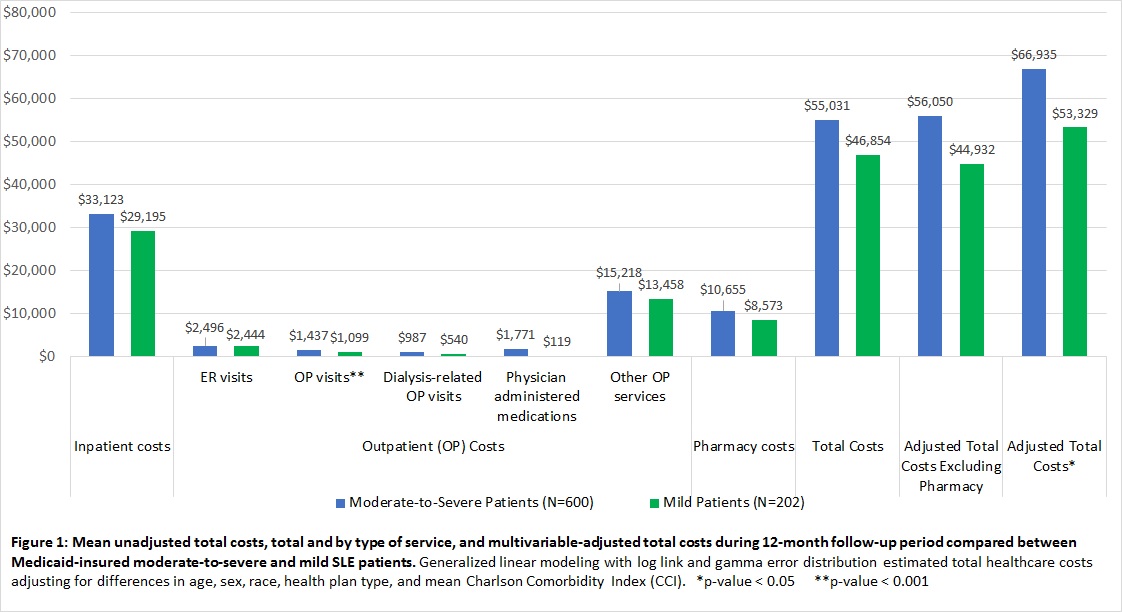
Results: 802 treated SLE patients were identified (91.3% female; 56.1% black). Based on patients’ SLE treatment, 74.8% were classified as having moderate-to-severe SLE. Patients with moderate-to-severe disease were significantly younger than those with mild disease (mean age [SD]: 41.4 years [12.4] vs. 46.5 years [9.9]; p<0.001) and were more likely to have lupus nephritis (34.2% vs. 18.1%; p<0.001). Mean unadjusted total costs and by type of service, and multivariable-adjusted total costs are shown in Figure 1. Over half of the total costs were derived from the inpatient setting. The mean multivariable-adjusted total costs incurred over 12 months of follow-up were $66,935 among moderate-to-severe patients and $53,329 among mild patients (p=0.04); mean multivariable-adjusted total costs excluding pharmacy costs were $56,050 among moderate-to-severe patients and $44,932 among mild patients (p=0.06).
Conclusion: Approximately ¾ of SLE patients within this Medicaid cohort received treatment indicative of moderate-to-severe SLE indicating a substantial burden of disease. Healthcare costs were high for all patients, and the greatest proportion of cost was due to inpatient admissions.
To cite this abstract in AMA style:
Costenbader K, Kabadi S, Durden E, Winer I, Bacani K, Yazdany J, Clarke AE. The Economic Burden of Systemic Lupus Erythematosus (SLE) within a Medicaid Cohort Stratified By Disease Severity [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/the-economic-burden-of-systemic-lupus-erythematosus-sle-within-a-medicaid-cohort-stratified-by-disease-severity/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-economic-burden-of-systemic-lupus-erythematosus-sle-within-a-medicaid-cohort-stratified-by-disease-severity/