Session Information
Session Type: ACR/ARHP Combined Abstract Session
Session Time: 9:00AM-11:00AM
Background/Purpose: Most currently available estimates of the economic burden of SLE in the US do not consider disease severity. The purpose of this study is to compare the economic burden between patients receiving treatment indicative of mild versus moderate-to-severe SLE.
Methods: Individuals ages 18-64 years, with an SLE ICD-9-CM code, treated with antimalarials, biologics (abatacept, belimumab, and rituximab), immunosuppressants, or systemic glucocorticoids from 1/1/2010 through 12/31/2014 were identified in a large administrative claims database. The first prescription fill date was the index date. Patients were required to have ≥ 1 inpatient claim or 2 non-diagnostic outpatient claims >30 days apart for SLE in the 12 months prior to index. If the patient had only outpatient claims, ≥ 1 SLE diagnoses must have been made by a rheumatologist or nephrologist. Patients were categorized according to SLE treatment during a 6-month exposure period after the index as: 1) mild SLE: either antimalarial or low-dose oral glucocorticoid (<=5 mg/day) monotherapy or 2) moderate-to-severe SLE: any immunosuppressive or combinations of SLE medications other than either antimalarial or low-dose oral glucocorticoid monotherapy. All-cause healthcare utilization and costs were evaluated during the 12 months following the initial exposure period (in 2016 US dollars). Generalized linear modeling with log link and gamma error distribution estimated: 1) total costs and 2) total costs excluding outpatient pharmacy costs during follow-up, adjusting for demographic and clinical characteristics.
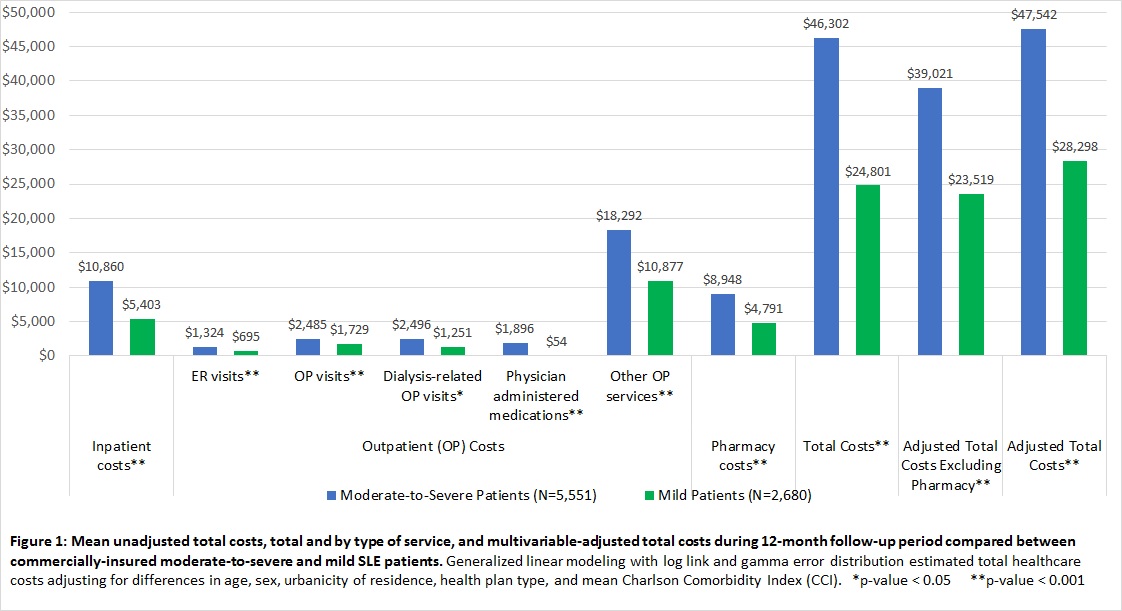
Results: 8,231 treated SLE patients were identified; based on treatment during the exposure period, 32.6% were classified as having mild SLE (mean age [SD]: 47.7 years [10.2]; 92.2% female) and 67.4% as having moderate-to-severe SLE (mean age [SD]: 46.6 years [10.6]; 90.2% female). Patients receiving treatment indicative of moderate-to-severe SLE had significantly (p<0.001) more lupus nephritis (20.2% vs. 8.1%), and more comorbidities including cardiovascular disease (13.3% vs. 10.3%), type 2 diabetes (10.3% vs. 6.9%), and hypertension (41.9% vs. 34.0%). Mean unadjusted total costs and by type of service and multivariable adjusted total costs are shown in Figure 1. The mean multivariable-adjusted total costs and total costs excluding pharmacy costs, respectively, were $47,542 and $39,021 among moderate-to-severe patients and $28,298 and $23,519 among those with mild SLE (p<0.0001 for both).
Conclusion: The mean 12-month adjusted total costs for patients with moderate-to-severe SLE were 68% higher than those of patients with mild SLE. These differential costs are important to consider in future studies examining cost-effectiveness and in designing interventions to improve health and reduce health spending for SLE.
To cite this abstract in AMA style:
Clarke AE, Kabadi S, Durden E, Winer I, Bacani K, Costenbader K, Yazdany J. The Economic Burden of Systemic Lupus Erythematosus (SLE) within a Commercially-Insured Population in the United States Stratified By Disease Severity [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/the-economic-burden-of-systemic-lupus-erythematosus-sle-within-a-commercially-insured-population-in-the-united-states-stratified-by-disease-severity/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-economic-burden-of-systemic-lupus-erythematosus-sle-within-a-commercially-insured-population-in-the-united-states-stratified-by-disease-severity/