Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: APS ACTION “Registry” was created to study the long-term natural history and outcomes of persistently antiphospholipid antibody (aPL)-positive patients with and without other systemic autoimmune diseases (SAID). Our primary objective was to describe baseline demographic, clinical, and laboratory characteristics of patients enrolled since 2010.
Methods: A web-based data capture system is used to store patient demographics and aPL-related medical history. APS ACTION Registry includes adults aged 18 to 60 years with positive aPL, based on the Updated Sapporo APS Classification Criteria, tested on two occasions at least twelve weeks apart within one year prior to enrollment. Patients are followed every 12±3m with clinical data and blood collection. For this retrospective analysis, we descriptively evaluated the sociodemographic and clinical characteristics of patients overall and in categories by disease manifestation (aPL positive without APS, thrombotic APS, and obstetric APS). We also assessed the clinical characteristics of aPL-positive patients who were tested for all three aPL (lupus anticoagulant [LA] test, anticardiolipin antibody [aCL], and anti-β2-Glycoprotein-I [aβ2GPI]) by subgroups based on aPL profiles (LA only, single, double, and triple positivity). Positivity for aCL IgG/M/A and aβ2GPI IgG/M/A was defined as a titer of ≥40 GPL/MPL/APL units and the highest titer among all test results was taken into consideration during analysis.
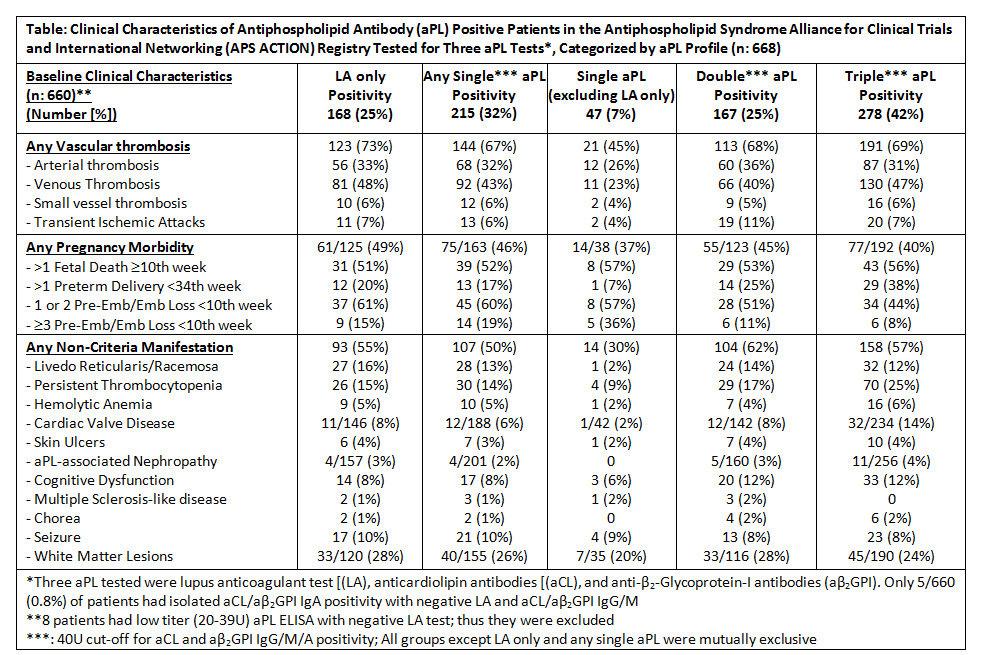
Results: As of March 2019, 804 patients were enrolled from 26 centers (mean age: 45 ± 13y; female: 74%; white 68%; and other SAID: 36%). Overall, 172 (21%) were aPL-positive without APS, 453 (56%) thrombotic APS; 73 (9%) purely obstetric APS; and 106 (13%) both thrombotic and obstetric APS. Among thrombotic APS patients, there were slightly higher rates of venous vs arterial thrombosis (62% vs 49%). Slightly higher rates of at least one non-criteria manifestation were observed in APS versus aPL only patients (58% vs 49%). All three aPL were tested in 668 (83%) patients, of whom 278 (42%) were triple positive. While similar frequencies of overall vascular thrombosis, pregnancy morbidity, and non-criteria manifestation were seen across single, double, and triple positivity subgroups, the lowest frequencies were observed in the single aPL positivity (excluding lupus anticoagulant [LA] only) subgroup (Table).
Conclusion: One-fifth of APS ACTION patients do not fulfill clinical APS classification criteria; 70% have vascular events; one-fifth have obstetric morbidity; and more than half have at least one non-criteria manifestation. Within single aPL-positivity, LA positivity appears to be an important contributor to aPL-related clinical features. Future prospective analyses, using standardized core laboratory aPL tests, will help better clarify aPL risk profiles.
To cite this abstract in AMA style:
Sevim E, Zisa D, Andrade D, Pengo V, Sciascia S, Tektonidou M, Ugarte A, Gerosa M, Belmont H, Lopez Pedrera R, Ji L, Fortin P, Efthymiou M, De Jesus G, Branch D, Andreoli L, Petri M, Cervera R, Rodriguez E, Knight J, Atsumi T, Willis R, Bertolaccini M, Cohen H, Roubey R, Erkan D, Barbhaiya M, APS ACTION o. The Clinical and Laboratory Characteristics of Antiphospholipid Antibody Positive Patients Included in the AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Clinical Database and Repository (“Registry”) [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-clinical-and-laboratory-characteristics-of-antiphospholipid-antibody-positive-patients-included-in-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-acti/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-clinical-and-laboratory-characteristics-of-antiphospholipid-antibody-positive-patients-included-in-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-acti/