Session Information
Date: Tuesday, November 12, 2019
Title: Pediatric Rheumatology – ePoster III: Systemic JIA, Fever, & Vasculitis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: There continues to be uncertainty regarding when to start biologic medications for polyarticular juvenile idiopathic arthritis (P-JIA). The Childhood Arthritis and Rheumatology Research Alliance (CARRA) developed consensus treatment plans (CTPs) that reflect the most commonly-used strategies for starting biologic treatment in untreated P-JIA patients. The CARRA STOP-JIA study compares the effectiveness of the CARRA CTPs in achieving clinical inactive disease (CID) at 12 months using a prospective, observational study design. Enrollment was completed in August 2018.
Methods: Untreated P-JIA patients with ≥ 5 active joints were enrolled into the CARRA Registry. One of the CARRA P-JIA CTPs was chosen by the physician and patient/family to follow without randomization or blinding: 1) Step-Up (begin with non-biologic DMARD and add biologic after 3 months if needed); 2) Early Combination (begin both DMARD and biologic at baseline); and 3) Biologic First (biologic monotherapy). Providers prescribed glucocorticoids per their usual practice. Patient Reported Outcomes (PROs) included the CHAQ, Patient/Parent Global Assessment, PROMIS® Pain Interference and Mobility measures.
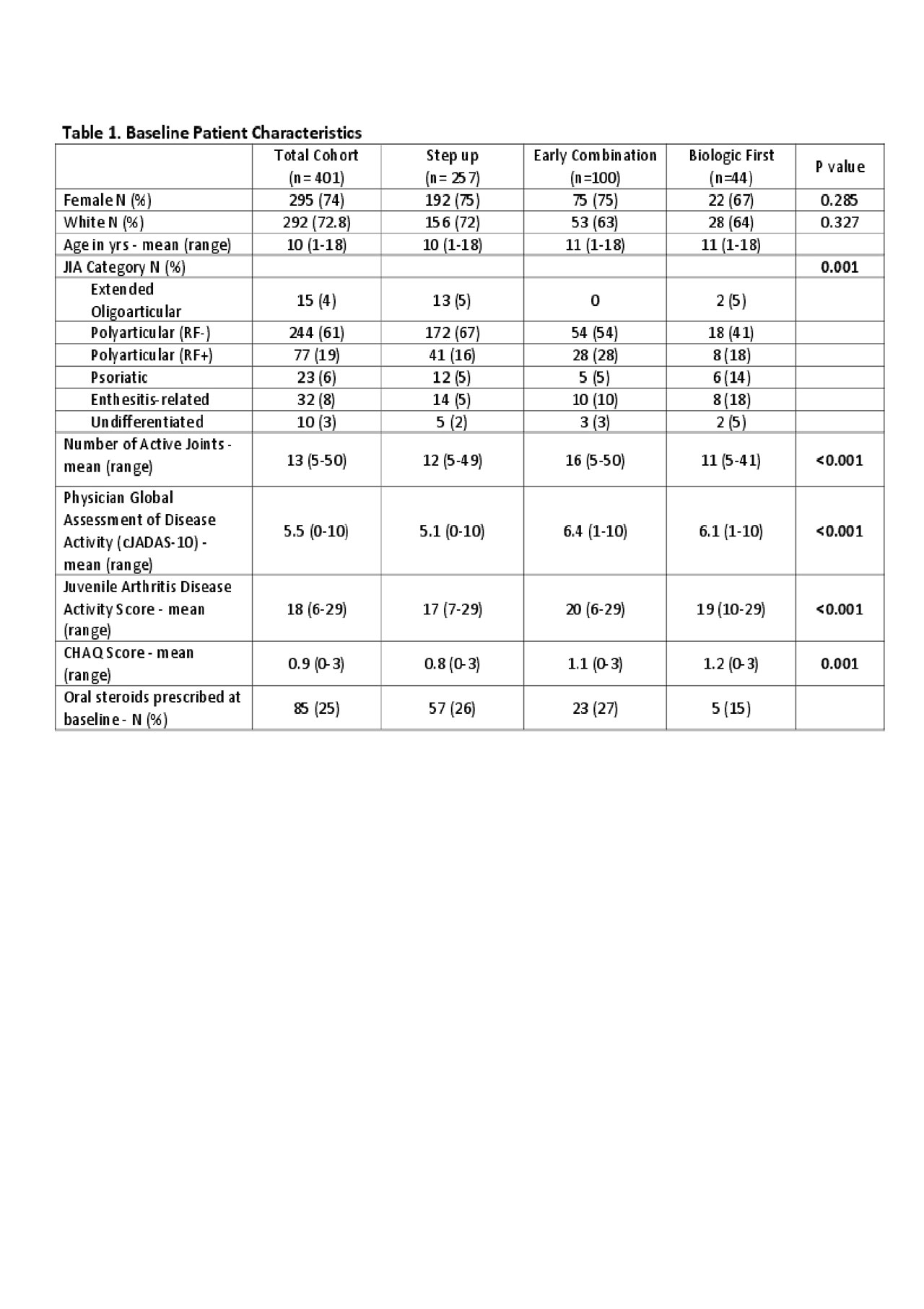
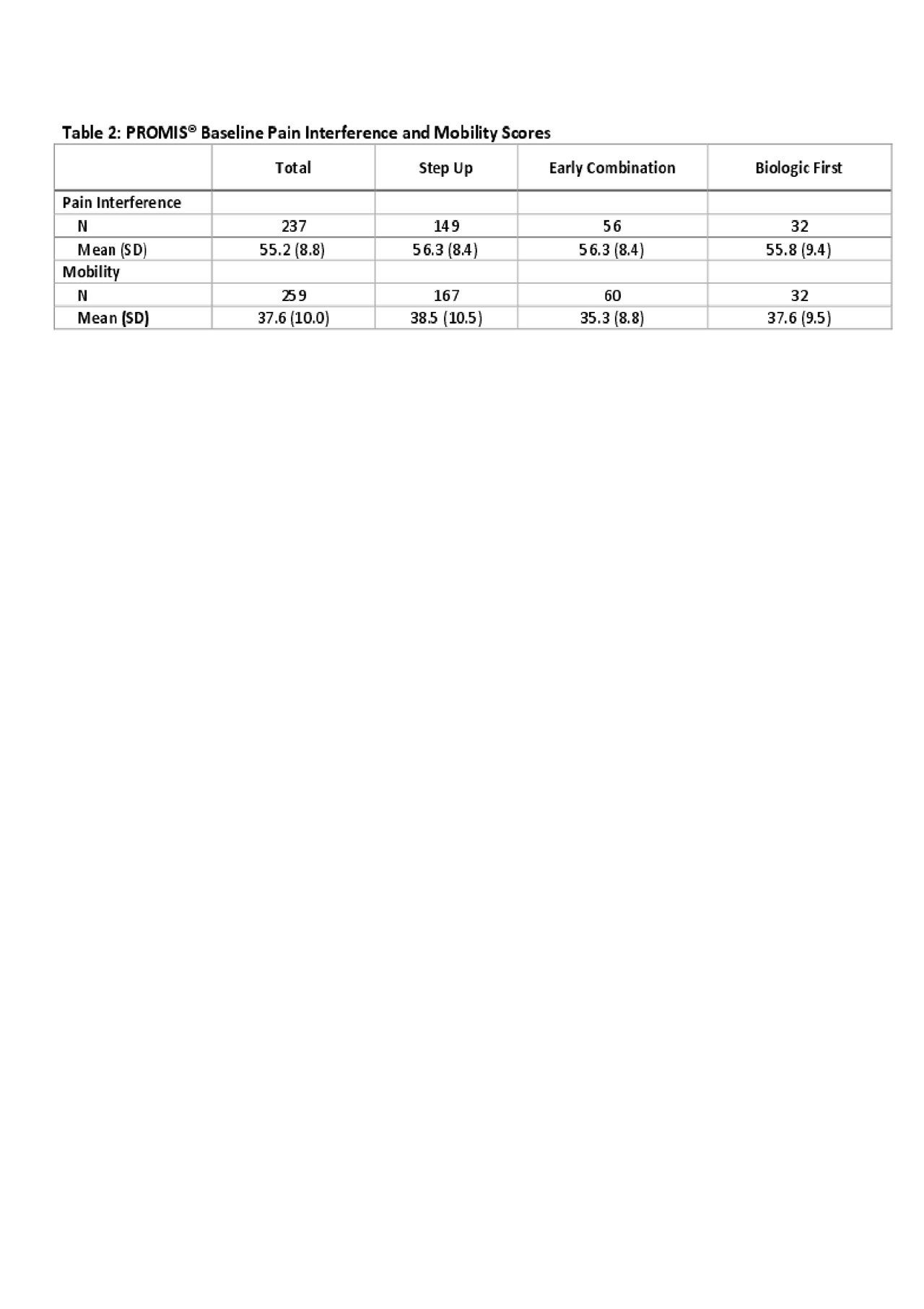
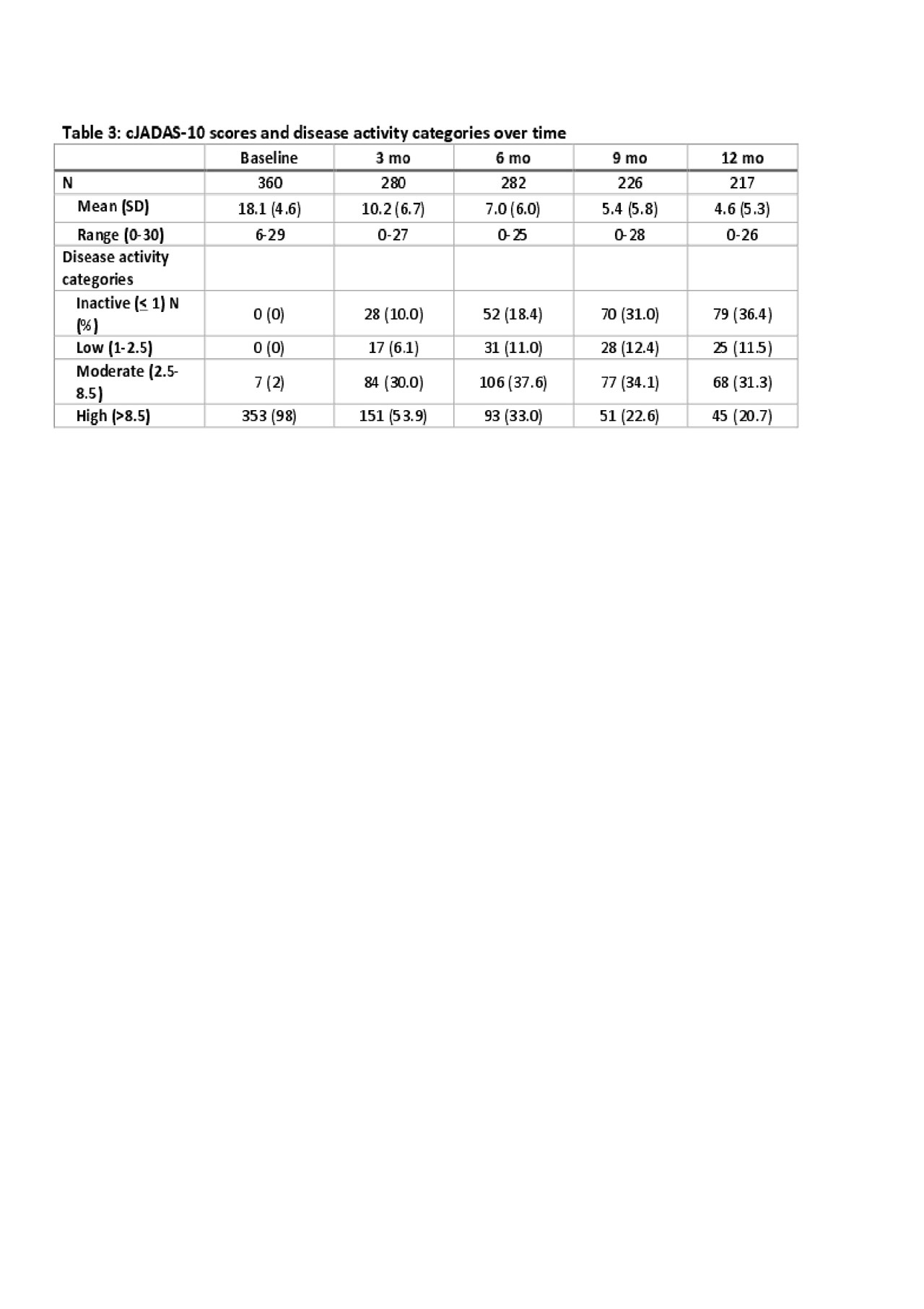
Results: Four hundred and one patients were enrolled at 44 sites in the US and Canada between 11/2015 and 8/2018. Table 1 shows the baseline data. The most commonly chosen CTPs were Step-Up (n=257; 64%) and Early Combination (n=100; 25%). At baseline, 343 (86%) of patients were started on a non-biologic DMARD (methotrexate). 147 (37%) were started on a biologic DMARD, most commonly etanercept (n=103, 26%) and adalimumab (49, 13%). To date, 320 patients have completed 12-months follow up. 278 patients completed at least one PRO measure at baseline. Table 2 shows mean PROMIS® Pain Interference and Mobiity scores for each treatment group. Table 3 shows the clinical Juvenile Arthritis Disease Activity Scores based on 10 joints (cJADAS-10) and the percentage of participants in each disease activity score category (inactive, low, moderate and high) over time.
Conclusion: STOP-JIA has successfully completed enrollment. Patients enrolled into all CTP choices; however, the Step-Up CTP was most common. There are differences between the treatment groups at baseline, including in PROMIS® measures, which will require statistical adjustment to reduce bias. Overall, disease activity reduced across all treatment groups over time. Once all patients have completed 12-month follow-up, analyses comparing the effectiveness of the different CTP strategies will be performed.
To cite this abstract in AMA style:
Ringold S, Tomlinson G, Weiss P, Schanberg L, Riordan M, Dennos A, Del Gaizo V, Murphy K, Feldman B, Kimura Y. The Childhood Arthritis and Rheumatology Research Alliance Start Time Optimization of Biologic Therapy in Polyarticular JIA Study: Patient Characteristics, Patient Reported Outcomes and Consensus Treatment Plan Choices [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-childhood-arthritis-and-rheumatology-research-alliance-start-time-optimization-of-biologic-therapy-in-polyarticular-jia-study-patient-characteristics-patient-reported-outcomes-and-consensus-trea/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-childhood-arthritis-and-rheumatology-research-alliance-start-time-optimization-of-biologic-therapy-in-polyarticular-jia-study-patient-characteristics-patient-reported-outcomes-and-consensus-trea/