Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Leukopenia is a common dose-dependent side effect of azathioprine and often results in discontinuation of the drug. Variants in TPMT and NUDT15 have been associated with leukopenia and tests are available for use in routine clinical practice. These tests can be used to ascertain patients’ metabolizer status. However, only 25% leukopenia cases associated with azathioprine use can be attributed to these genetic variants.
Methods: We used BioVU, Vanderbilt’s electronic health record linked to genetic data, to assemble a discovery cohort of new users of azathioprine. The analysis was conducted on a group of 1184 patients whose reported (self or healthcare provider) race was White, were TPMT/NUDT15 normal metabolizers, and had no history of thiopurine use or organ transplantation. Using similar inclusion/exclusion criteria, our replication cohort consisted of 521 self-reported White patients enrolled in All of Us – one of the largest and most diverse research programs in the U.S., with data including electronic healthcare records and linked DNA. Leukopenia was defined as a white blood cell count less than or equal to 3 x 10 9/L. We performed genome-wide association analyses and results were adjusted for sex, age at event (age at last dose if control), last dose, 4 indication variables (non-SLE inflammatory conditions, inflammatory bowel disease, SLE, and other), concurrent xanthine oxidase inhibitor (allopurinol and febuxostat) use, concurrent immunosuppressant use, prior TPMT genetic or enzymatic testing, and 10 genetic principal components.
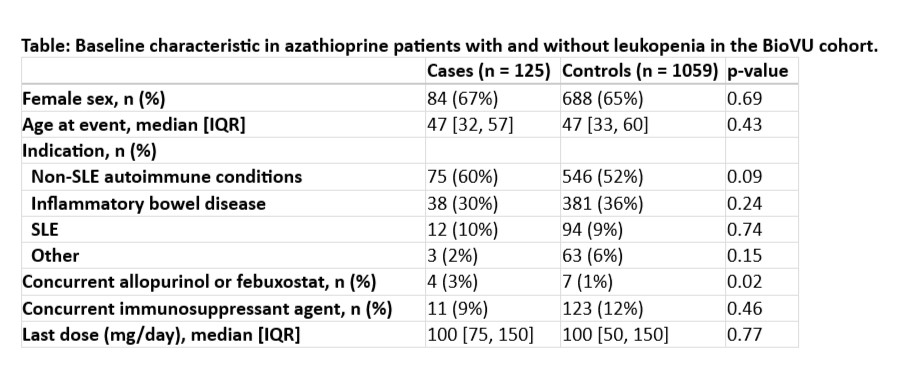
Results: The BioVU cohort was predominantly female and median age was 47 (Table). There were 125 cases of patients who developed leukopenia while on azathioprine treatment in the BioVU cohort. The All of Us cohort was also predominantly female, and the median age was 52; there were 477 control and 44 cases. In the BioVU cohort, we found two variants, rs11664064 and rs45566135, in the PTPN2 locus that reached genome-wide significance with odds ratios of 3.61 (p=1.964e-08) and 3.65 (p=1.602e-08), respectively. In the All of Us cohort, both variants replicated (rs11664064: OR = 2.94, p=0.051 and rs45566135: OR = 2.94, p=0.027). The two variants were determined to be in high linkage disequilibrium in both cohorts (R2 = 0.98).
Conclusion: Using a genome-wide approach we identified an association between variants in the PTPN2 locus and leukopenia among new users of azathioprine. Our results align with prior reports that indicate that PTPN2 regulates the development and differentiation of immune cells.
To cite this abstract in AMA style:
Nepal P, Daniel L, Zanussi J, Dickson A, Wei W, Hung A, Cox N, Kawai V, Mosley J, Stein C, Feng Q, Liu G, Tao R, Chung C. The Association Between PTPN2 and Leukopenia in New Users of Azathioprine [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/the-association-between-ptpn2-and-leukopenia-in-new-users-of-azathioprine/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-association-between-ptpn2-and-leukopenia-in-new-users-of-azathioprine/