Session Information
Date: Sunday, November 13, 2016
Title: Vasculitis - Poster I: Large Vessel Vasculitis and Polymyalgia Rheumatica
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Methods: 30 patients with Giant Cell Arteritis (GCA) were randomized in this RCT in a 2:1 ratio into receiving intravenously (i.v.) TCZ 8mg/kg bodyweight plus Glucocorticoids (GC) or Placebo (PB) plus GC. They received infusions in 4-weekly intervals for 52 weeks. Thereafter TCZ medication was stopped, further treatment was prescribed by the treating physicians, patients were followed up clinically.
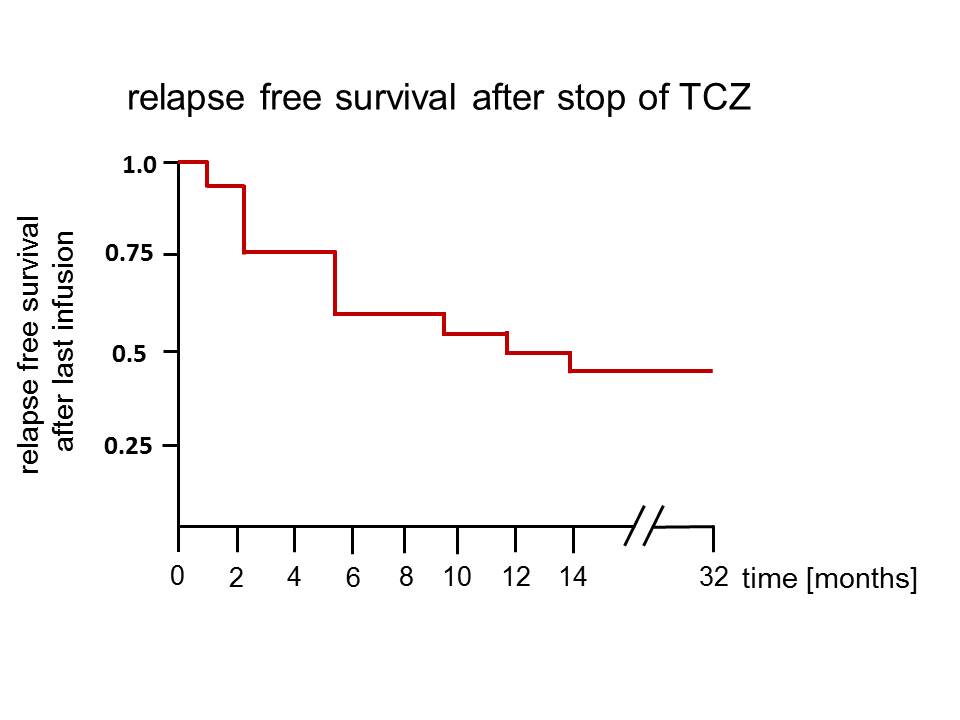
Results: By week 52 of the RCT all patients of the TCZ arms were in sustained complete remission, of these 18 without GC co-medication. 2/20 patients received GC after the last infusion due to premature stop of TCZ, one patient with Stevens-Johnson-syndrome and one with diverticulitis. Median follow-up time was 12.5 months (range 3-32). After the last infusion of TCZ 11/20 patients relapsed with a median time to relapse of 5 months (range 2-14). In the placebo arm all but one patient relapsed and/or continued GC treatment. Remarkably, 1/10 PB patients remained in remission throughout the study and was without medication at last follow-up, 10 months after the end of study. None of the relapsing patients experienced blindness, aortic rupture, aortic stenosis or other major vascular complications. In case of relapse, dose of GC was 1mg/kg bodyweight in signs of major relapse and 20-40 mg/d in minor relapse according to the average dose during the study period that was sufficient to control symptoms in PB patients prior to relapse. Additionally, in 6/11 TCZ patients relapsing after the last study infusion, TCZ was re-administered with 8mg/kg bodyweight i.v. in monthly intervals after a median time of 6.5 months (range 3-14). In 2/6 patients with TCZ re-introduction, TCZ was stopped after 4 and 6 months, respectively, with lasting remission. In 1/6 patients TCZ was again given for 2 months, stopped in remission yet had to be re-introduced 6 months later due to a second relapse.
Conclusion: Clinical and serologic remission in response to TCZ for 52 weeks does not result in relapses-free survival after termination of treatment. Although IL-6 blockade using TCZ controls clinical disease, it may not control pathogenesis in all cases. The fact that 45% of patients remained in lasting remission may help to design treatment protocols to determine appropriate maintenance dosage regimens of TCZ after achievement of remission.
To cite this abstract in AMA style:
Adler S, Reichenbach S, Kuchen S, Wermelinger F, Dan D, Seitz M, Villiger PM. Termination of Tocilizumab-Treatment in Giant Cell Arteritis: Follow-up of Patients after the RCT (ClinicalTrials.gov registration number: NCT01450137) [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/termination-of-tocilizumab-treatment-in-giant-cell-arteritis-follow-up-of-patients-after-the-rct-clinicaltrials-gov-registration-number-nct01450137/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/termination-of-tocilizumab-treatment-in-giant-cell-arteritis-follow-up-of-patients-after-the-rct-clinicaltrials-gov-registration-number-nct01450137/