Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
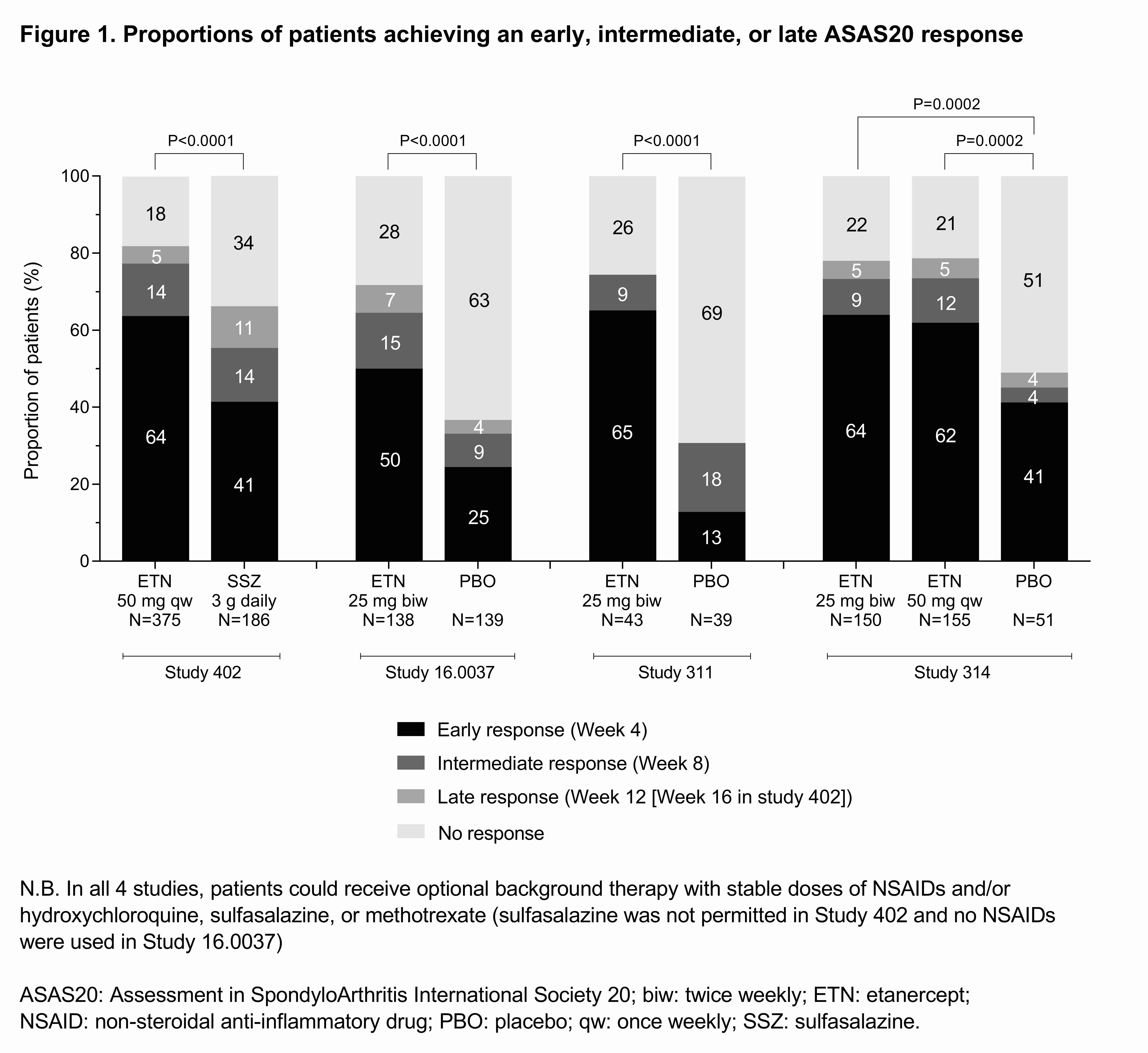
Background/Purpose: Treatment with etanercept (ETN) is effective and well tolerated in patients with axial spondyloarthritis (AS), but the time frames within which patients tend to achieve clinical responses have not been well characterized. The aim of this post hoc analysis was to compare the temporal achievement of initial Assessment in SpondyloArthritis International Society 20 (ASAS20) response or initial inactive disease (ID) status in patients randomized to the ETN or control arms of clinical trials.
Methods: Data from 4 multicenter, double-blind, randomized, placebo- or sulfasalazine-controlled trials evaluating the safety and efficacy of ETN in patients with AS were included: Study 402 (phase 4, N=566; NCT00247962), Study 16.0037 (phase 3, N=277; NCT00356356), Study 311 (phase 3, N=84; NCT00421915), and Study 314 (phase 3, N=350; NCT00418548). The proportion of patients achieving ASAS20 response was the primary endpoint in all 4 studies and each study involved patients receiving ≥12 weeks of ETN treatment. In all 4 studies, patients could receive optional background therapy with stable doses of NSAIDs and/or DMARDs. Analyses were based on observed cases, with the first incidence of each patient achieving an ASAS20 response or ID status (Ankylosing Spondylitis Disease Activity Score with C-reactive protein [ASDAS-CRP] < 1.3) categorized as early, intermediate, or late depending on whether it occurred at Week 4, Week 8, or Week 12 (Week 16 for Study 402), respectively. Patients not achieving ASAS20 response or ID status by Week 12/Week 16 were categorized as “no response”. Proportions of patients achieving early, intermediate, late, or no response in the different treatment arms within each study were compared using Cochran–Mantel–Haenszel tests without adjustment for multiplicity.
Results: Overall, analyses of ASAS20 response and ID status included data from 1276 and 1261 patients, respectively. Within each of the 4 studies, the proportion of patients who achieved either early ASAS20 response (Figure 1) or early ID status (Figure 2) was significantly greater in the ETN arm compared with the control arm. Across the 4 studies, 50–65% of patients in the ETN arms and 13–41% of patients in the control arms had an early ASAS20 response, with smaller proportions achieving intermediate or late ASAS20 responses. For the analysis of ID status, 7–24% of patients in the ETN arms and ≤4% of patients in the control arms achieved early ID status across the 4 studies, with similar proportions achieving intermediate or late ID status.
Conclusion: The proportion of patients with no response to treatment was smaller in the ETN arms than the control arms throughout all 4 studies analyzed, with the superior clinical response to ETN predominantly driven by significantly greater proportions of the ETN-treated patients achieving an early ASAS20 response or early ID status. The achievement of ASAS20 response showed a different temporal pattern to the achievement of ID status. The majority of ASAS20 responses were categorized as early whereas similar proportions achieved ID status categorized as early, intermediate, or late.
To cite this abstract in AMA style:
Baraliakos X, Szumski A, Kwok K, Vlahos B. Temporal Achievement of Clinical Response and Inactive Disease Status in Patients with Axial Spondyloarthritis Treated with Etanercept [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/temporal-achievement-of-clinical-response-and-inactive-disease-status-in-patients-with-axial-spondyloarthritis-treated-with-etanercept/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/temporal-achievement-of-clinical-response-and-inactive-disease-status-in-patients-with-axial-spondyloarthritis-treated-with-etanercept/