Session Information
Date: Tuesday, November 9, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster III: Psoriatic Arthritis II (1801–1835)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Guselkumab (GUS), a human monoclonal antibody that specifically binds to the p19 subunit of IL-23, demonstrated efficacy and a favorable safety profile in active PsA in the Phase (Ph) 21 and Ph3 DISCOVER-1&2 trials2,3 and in moderate-to-severe plaque psoriasis (PsO) in the Ph3 VOYAGE-1&2 trials.4,5
Methods: Using pooled safety data through 2 years (yrs) from the PsA trials (N=1229; GUS 100 mg every 4 or 8 weeks [Q4W/Q8W])1-3 and through 5 yrs from the PsO trials (N=1721; GUS 100 mg Q8W),4,5 the incidences of serious adverse events (SAEs); gastrointestinal (GI)-related SAEs and other targeted AEs; including candidiasis, uveitis, and opportunistic infections (OIs; e.g., active tuberculosis [TB]) were evaluated. GI-related SAEs were identified using the Medical Dictionary for Regulatory Activities (MedDRA) system-organ class “GI disorders;” OIs, uveitis/iridocyclitis, and candidiasis were classified through medical review of preferred terms and/or a specified MedDRA search strategy. Patients (pts) with a history of IBD were not excluded in the PsA/PsO trials. Rates of overall SAEs, GI-related SAEs, and other targeted AEs were calculated as the number of events per 100 pt-yrs of follow-up (PY), along with 95% confidence intervals (CI). Pooled safety data are presented for the placebo (PBO)-controlled periods (W0-16: PsO trials; W0-24: PsA trials) and active treatment periods through 2 yrs in PsA trials (W56: Ph2; W60: DISCOVER-1; W112: DISCOVER-2) and through 5 yrs in PsO trials (W264: VOYAGE-1&2). Maximum duration of exposure was W100 for the PsA trials and W252 for the PsO trials.
Results: The PsA and PsO populations had comparable mean age and BMI. Prior or concomitant medication use reflected standard of care and study entry criteria (Table 1). Incidence rates of SAEs and GI-related SAEs were generally similar between GUS- and PBO-treated pts during the PBO-controlled periods, and between PsA pts receiving GUS Q4W or Q8W for up to 2 yrs and PsO pts receiving GUS Q8W for up to 5 yrs (Tables 2,3). Rates of other targeted AEs of interest were low in GUS-treated PsA/PsO pts. OIs did not occur in PsO pts and were infrequent in PsA pts (1 case each of herpes zoster disseminated, fungal oesophagitis, meningitis listeria). Candidal infections were reported infrequently and were non-serious (Tables 2,3). Iridocyclitis was reported in 1 PBO-treated PsA pt and 1 GUS Q8W-treated PsA pt. No cases of exacerbations or new onset of IBD were reported in GUS-treated PsA/PsO pts. No cases of active TB occurred in GUS-treated PsA/PsO pts.
Conclusion: Incidence rates of SAEs; GI-related SAEs; and AEs of interest including candidiasis, uveitis, and OIs were low, or no cases were reported. No new safety concerns were identified with GUS treatment through 2 yrs and 5 yrs of follow-up in the pooled PsA and PsO trials, respectively, supporting a durable and favorable GUS safety profile consistent between pts with active PsA and moderate-to-severe PsO.
References:
1. Deodhar A, et al. Lancet. 2018;391:2213-2224.
2. Deodhar A, et al. Lancet. 2020;395:1115-1125.
3. Mease PJ, et al. Lancet. 2020;395:1126-1136.
4. Blauvelt A, et al. J Am Acad Dermatol. 2017;76:405-417.
5. Reich K, et al. J Am Acad Dermatol. 2017;76:418-431.
 Table 1: Baseline Disease Characteristics and Medications
Table 1: Baseline Disease Characteristics and Medications
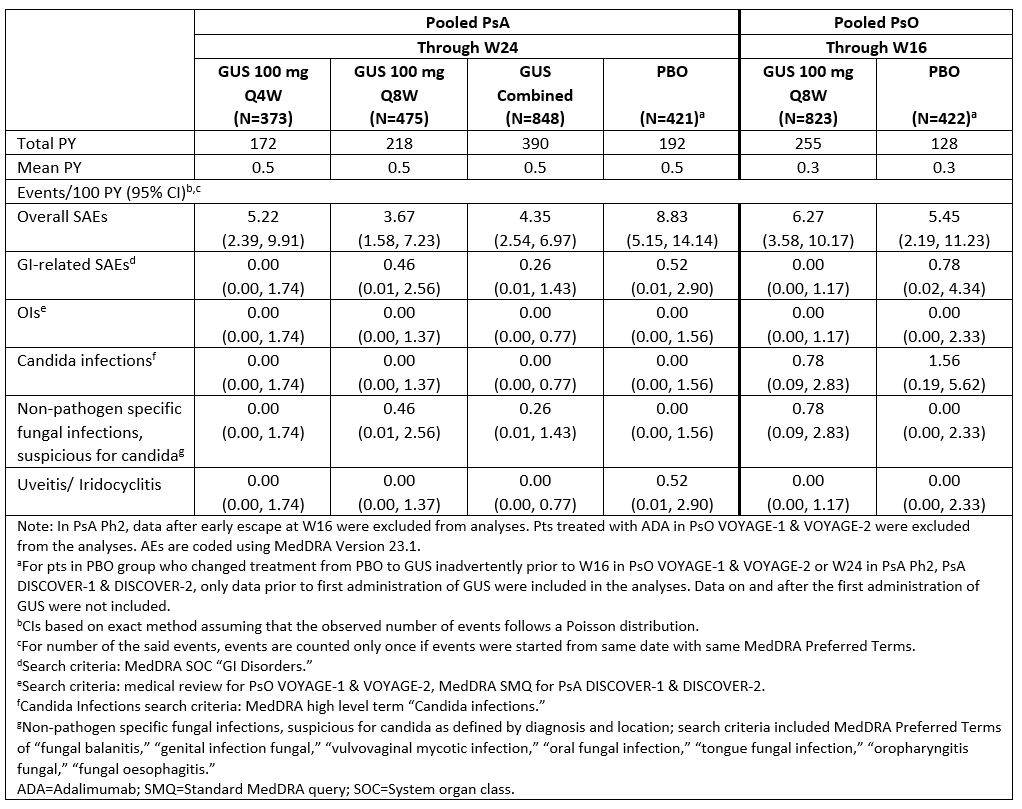 Table 2. Targeted AEs of Interest Through PBO-controlled Periods
Table 2. Targeted AEs of Interest Through PBO-controlled Periods
 Table 3: Targeted AEs of Interest Through End of Study
Table 3: Targeted AEs of Interest Through End of Study
To cite this abstract in AMA style:
Mease P, Foley P, Reich K, Chakravarty S, Shawi M, Yang Y, Miller M, Kollmeier A, Xu X, Yu J, Wang Y, Sheng S, You Y, McInnes I. Targeted Safety Analyses of Guselkumab (TREMFYA®): Long-term Results from Randomized Clinical Trials in Patients with Active Psoriatic Arthritis and Moderate to Severe Psoriasis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/targeted-safety-analyses-of-guselkumab-tremfya-long-term-results-from-randomized-clinical-trials-in-patients-with-active-psoriatic-arthritis-and-moderate-to-severe-psoriasis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/targeted-safety-analyses-of-guselkumab-tremfya-long-term-results-from-randomized-clinical-trials-in-patients-with-active-psoriatic-arthritis-and-moderate-to-severe-psoriasis/
