Session Information
Date: Sunday, November 12, 2023
Title: (0510–0542) Spondyloarthritis Including Psoriatic Arthritis – Treatment: AxSpA Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The recent recommendations from EULAR-ASAS for Axial Spondyloarthritis (AxSpA) call for decreasing biologic (b)DMARDs after an extended period of remission. This is especially relevant in underdeveloped countries, where patients pay out of pocket for their healthcare and are exposed to higher risks of opportunistic infections with bDMARD therapy. Despite sharing the same issues of infections and expenses, there is no clear evidence for the tapering strategy of targeted synthetic DMARDs in AxSpA. We aimed to study whether TOFA can be tapered in patients with AxSpa, assess the risk of flares, time to flare, and factors associated with flares.
Methods: Patients with AxSpA (ASAS criteria) on tofacitinib (TOFA) in remission or low disease activity (ASDAS CRP < 2.1) state for at least three months, were gradually tapered off TOFA. The drug was tapered at the treating physician’s discretion, with each visit accompanied by an evaluation of the disease activity. Co-treatment with conventional(c) DMARDs, but not bDMARDs was permitted. Tofacitinib was resumed at the previous dose in case of flares, defined at the treating physician’s discretion.
Flare-free survival was visualized by Kaplan-Meir curves. To determine factors associated with flares, covariates with a p-value of < 0.2 on univariate analysis (age, radiographic disease, cDMARD use in the past, ASDAS CRP > 3.5 at baseline) were included for multivariate Cox regression modelling. Data are expressed as median and interquartile range.
Results: The study included 54 patients, aged 38 years(29.75–46.75), 75% males, and a disease duration of 6 (9–17.5) years. Three-fourths had radiographic disease, and 30 of 44 (68.1%) were HLA-B27 positive. Amongs the extra-articular features,uveitis was observed in 9(16.7%).
Before TOFA initiation, ASDAS-CRP was 3.1(2.4-3.5), 24 (44.4%) had used cDMARDs, and 20 (37%) had used bDMARDs. Reasons for shifting to TOFA were an inadequate response to cDMARD or bDMARD in 36 (66.7%) cases; financial considerations with bDMARD therapy in 14 (26%), and adverse drug reactions in four (7.4%). TOFA was started at a full dose of 70 mg/week in 47 (87%), and 41 (76 %) received concomitant sulfasalazine but no bDMARDs were used with TOFA.
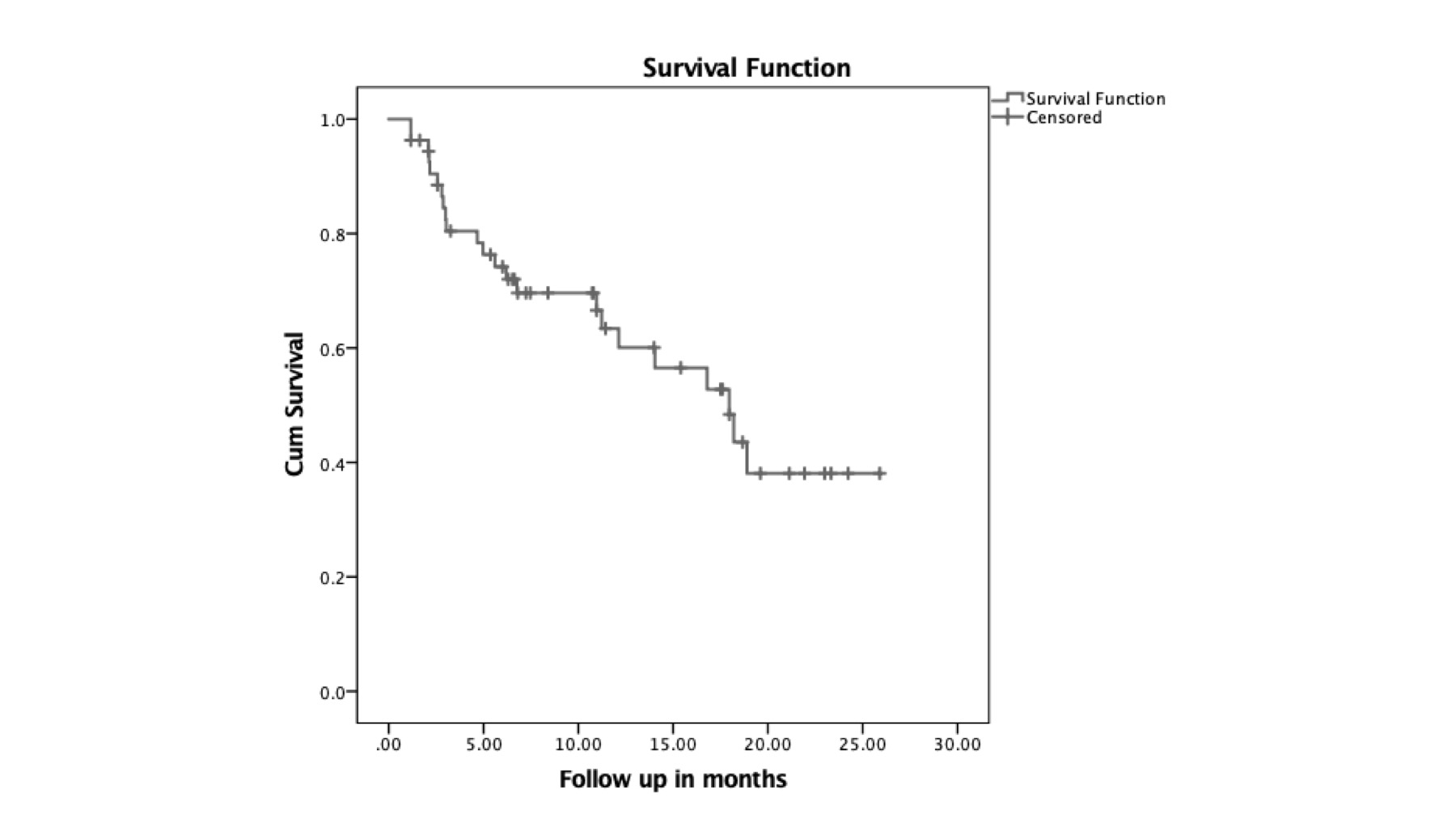
ASDAS CRP was 0.3 (0.9-1.7) at 3 months (3-5.25) of full dose TOFA. During tapering, 23 (42.6%) patients flared, with the median time to flare being 18 months (CI 12.5-23.4)(Figure 1,2). All patients who flared re-attained remission after restoring the TOFA dose to the previous dose.
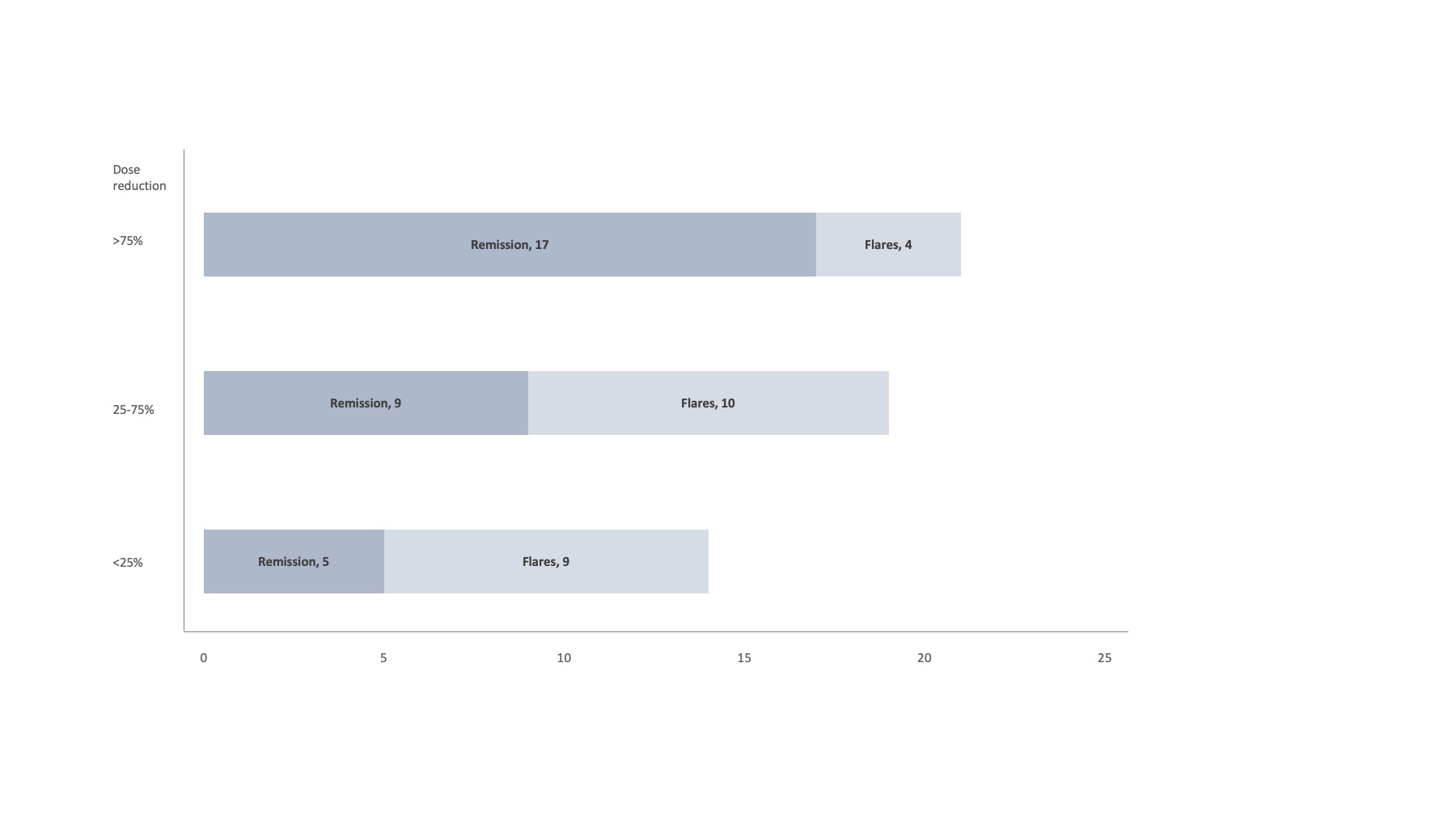
At a median of 7.3 months (3-17.4) of TOFA tapering, 39 (70%) were in remission with4 (10.5%) on >75%, 32( 84.2%) on 25-75% and 2(5.2%) on >25% of full TOFA dosage.
In multivariate Cox regression, radiographic disease [0.01, 7(1.6-31)], prior cDMARD [0.02, 0.3(0.1-0.8)] and ASDAS CRP >3.5 prior to initiation of TOFA [0.005, 3.4(1.4-8.1)] use were linked with flare.
Conclusion: This real-world data provides proof of concept that TOFA tapering is viable in the management of AxSpA. Flares can be managed by increasing the dose of TOFA to the previous effective level. This can pave the way for randomized controlled studies to determine the optimum tapering strategy, as well as long-term cohort studies to see the effects on radiographic progression.
To cite this abstract in AMA style:
Mehta P, Saijan S, George J, Ahmed S, Meghnathi B, Shenoy P. Tapering Tofacitinib for Axial Spondyloarthritis in Remission or Low-disease Activity State [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/tapering-tofacitinib-for-axial-spondyloarthritis-in-remission-or-low-disease-activity-state/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tapering-tofacitinib-for-axial-spondyloarthritis-in-remission-or-low-disease-activity-state/