Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: A vaccination coverage goal of 80% with the seasonal influenza vaccine was established to protect people at high-risk for influenza-related complications or hospitalizations (e.g. rheumatic diseases – RD). Inactivated influenza vaccine (IIV) coverage in RD patients remains below target. Vaccine hesitancy (i.e. delay in acceptance or refusal of vaccination despite availability of vaccination services) is a complex issue that threatens vaccine uptake. Understanding the reasons for influenza vaccine hesitancy among RD patients is fundamental to enhance vaccination coverage.
Methods: Between November and March 2020, we conducted a cross-sectional study of consecutive RD patients presenting to a rheumatology clinic at a Canadian university hospital. Patients indicated in a 10-point scale how likely they were to get the IIV. Three groups were defined: (a) refuse IIV (values 0-1-2), (b) accept IIV (values 8-9-10), and (c) uncertain (values 3-7). Demographic data, education, employment, RD diagnosis, treatment, and smoking status were compared between groups. Patients completed the WHO-Strategic Advisory Group of Experts (SAGE) questions on vaccine hesitancy (i.e. vaccine hesitancy determinants matrix). Multivariate logistical regression analyses were performed to evaluate predictors of vaccine hesitancy.
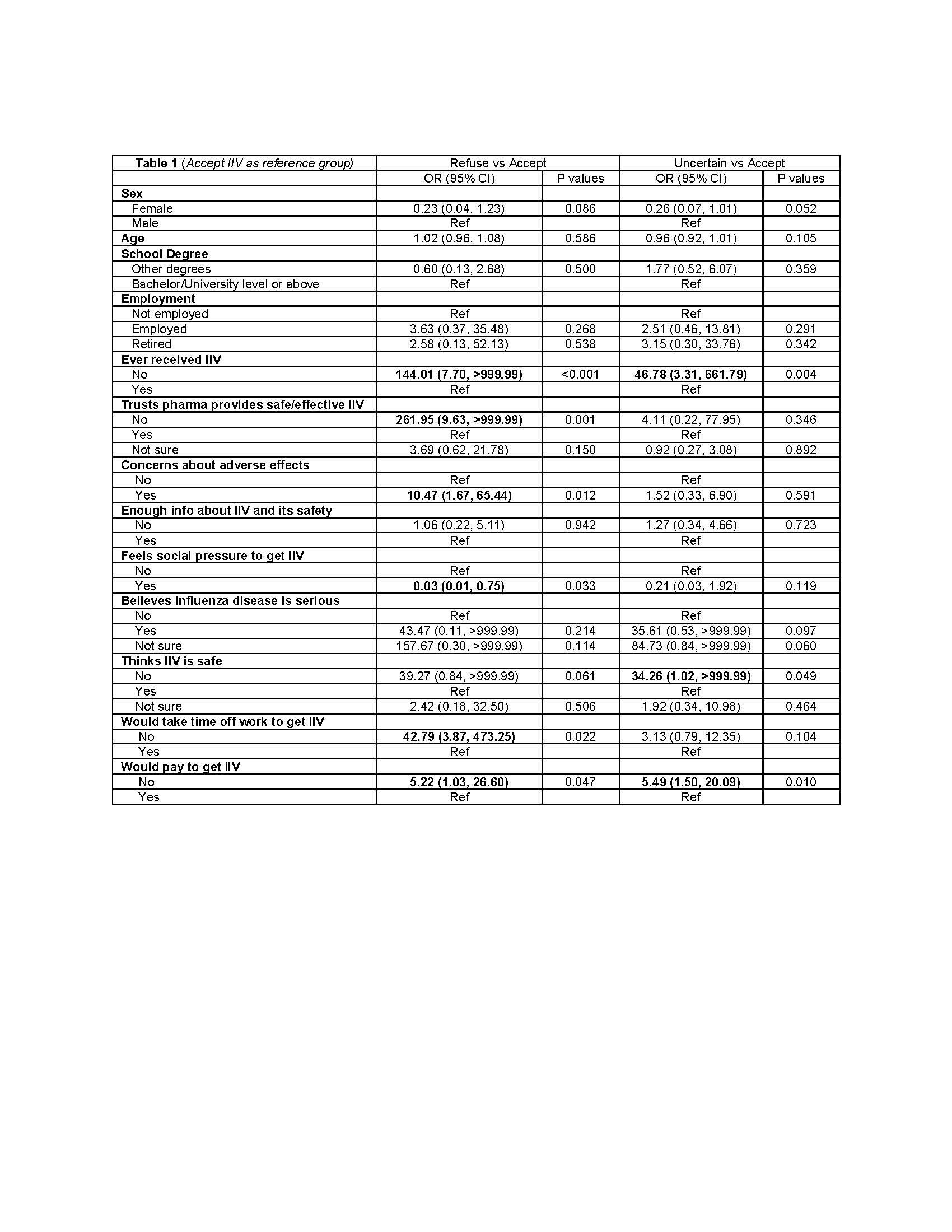
Results: 294 patients (101 rheumatoid arthritis-RA, 107 SLE/vasculitis-SARD, 55 spondyloarthropathies-SpA, 31 osteoarthritis-OA) were included in the analysis. Over a third them did not receive IIV in the previous year (RA 38.4%, SARD 39.1%, SpA 48.2%, OA 38.7%). According to how likely they were to get the IIV, three groups were defined: refused (n=67), accepted (n=165) and uncertain (n=40). Demographics, education, and smoking status did not differ between the three groups. Retired patients were more frequent among those who refused IIV (35% vs 12% in the uncertain group). The use of biologics was higher in the group that ‘accepted IIV’ (27% vs 13.4% in those who refused IIV). Reported reasons for hesitancy according to the WHO-SAGE matrix was similar across diseases. Table 1 summarizes the predictors of vaccine hesitancy.
Conclusion: Forty percent of RD patients, irrespective of their education, either refuse or express uncertainty about getting IIV. Concerns about adverse events and doubts that pharmaceutical companies are providers of safe and effective vaccines are key determinants of IIV hesitancy.
To cite this abstract in AMA style:
Valerio V, Useche M, Field E, Wang M, Hazel E, Ward B, Colmegna I. Systems Approach to Understanding Reasons for Influenza Vaccine Hesitancy in Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/systems-approach-to-understanding-reasons-for-influenza-vaccine-hesitancy-in-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/systems-approach-to-understanding-reasons-for-influenza-vaccine-hesitancy-in-rheumatic-diseases/