Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Post-hoc analysis of two Phase III studies of belimumab1 showed that an SRI(4) response is associated with clinically meaningful benefits, irrespective of treatment assignment. Confirmation of these findings in independent cohorts will enhance the acceptance of SRI(4) as a measure of clinically meaningful improvement. This analysis assessed global clinical benefit represented by an SRI(4) response.
Methods: Changes from baseline in clinical, laboratory, and patient-reported outcome measures at Day 365 were compared between SRI(4) responders (n=396) and non-responders (n=340) in the combined dataset of two Phase IIb studies evaluating sifalimumab and anifrolumab (MUSE) in moderate to severe SLE.
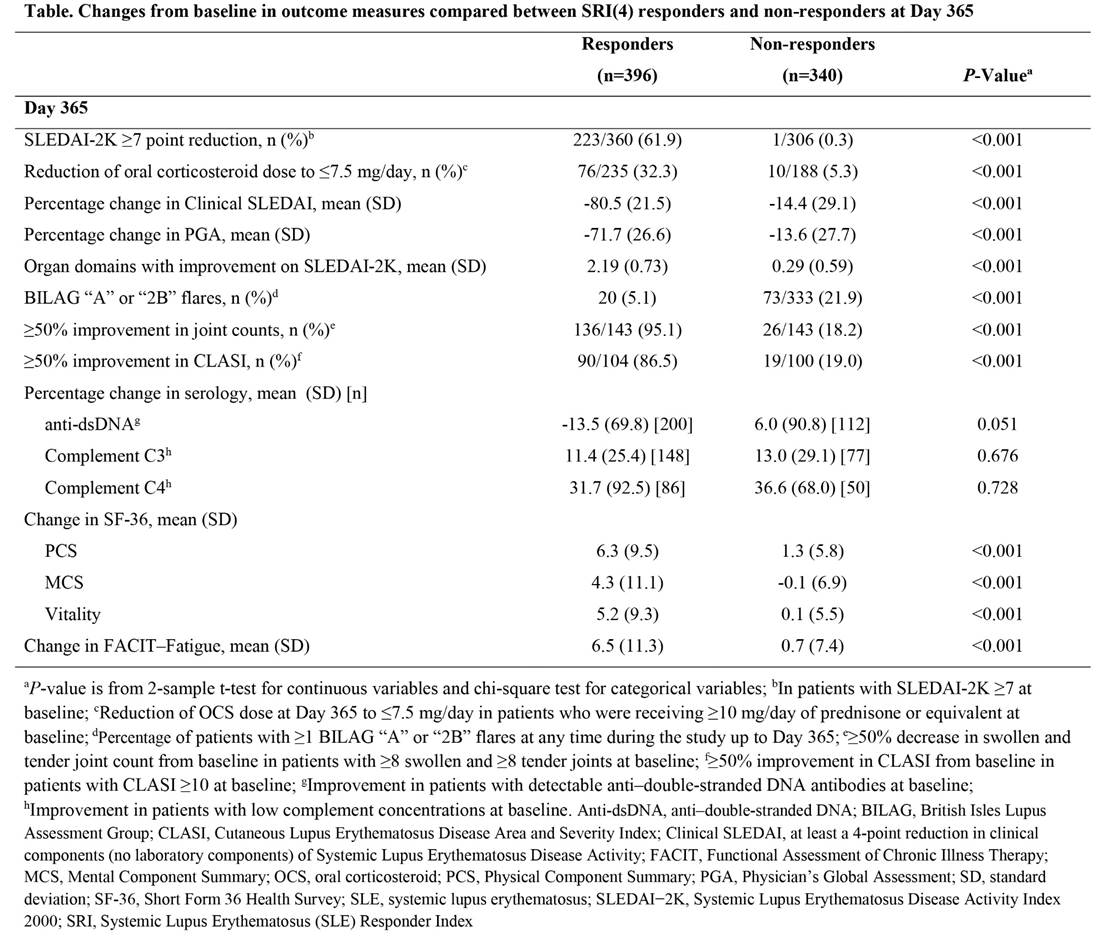
Results: Baseline demographics were similar between the studies. At Day 365, a greater percentage of responders than non-responders had ≥7-point reduction in SLE Disease Activity Index 2000 (SLEDAI-2K) and had their oral corticosteroid dose reduced to ≤7.5 mg/day (Table). Responders also had greater percentage changes from baseline in clinical SLEDAI scores, and greater improvements in Physician’s Global Assessment (PGA) score and number of SLEDAI-2K organ domains with improvement. British Isles Lupus Assessment Group (BILAG) “A” or “2B” flare rates were lower in SRI(4) responders. A larger percentage of responders with ≥8 swollen and ≥8 tender joints at baseline had ≥50% improvement in swollen and tender joint counts. In patients with moderate to severe skin disease at baseline, defined as Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) activity score ≥10, more responders had ≥50% improvement in CLASI. In patients with detectable anti–double-stranded DNA (anti-dsDNA) antibodies at baseline, responders had greater improvements in anti-dsDNA concentrations; however, in patients with low complement concentrations at baseline, the improvements in complement C3 and C4 concentrations were similar between responders and non-responders. Responders also had greater improvements in patient-reported outcomes: percentage change from baseline in Patient Global Assessment, and absolute change in Short Form 36 Health Survey (SF-36; Physical Component Summary, Mental Component Summary, Vitality) and Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue scores.
Conclusion: SRI(4) response in patients with moderate to severe SLE was associated with broad improvements in clinical and patient-reported outcomes. SRI(4) response in this analysis was driven by clinical components and confirm previous findings suggesting SRI(4) response is associated with global clinically important benefits. References: 1Furie R, et al. Lupus Sci Med 2014;1:e000031. First presented at EULAR 2016.
To cite this abstract in AMA style:
Furie R, Wang L, Drappa J, Illei G. Systemic Lupus Erythematosus (SLE) Responder Index [SRI(4)] Response Is Associated with Global Benefit in Patients with Moderate to Severe SLE [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/systemic-lupus-erythematosus-sle-responder-index-sri4-response-is-associated-with-global-benefit-in-patients-with-moderate-to-severe-sle/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/systemic-lupus-erythematosus-sle-responder-index-sri4-response-is-associated-with-global-benefit-in-patients-with-moderate-to-severe-sle/